Abstract
Insulin secretion rates are greater after oral glucose than after parenteral administration of an equivalent glucose load. This augmented β-cell secretory response to an oral glucose load results from the release of mainly two gut hormones: gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1, which potentiate glucose-induced insulin secretion. Because of their insulinotropic action, their abnormal secretion may be involved in the pathogenesis of the hyperinsulinemia of childhood obesity. In this study, we used the hyperglycemic clamp with a small oral glucose load to assess the effect of childhood obesity on GIP response in seven prepubertal lean and 11 prepubertal obese children and in 14 lean adolescents and 10 obese adolescents. Plasma glucose was acutely raised to 11 mM by infusing i.v. glucose and kept at this concentration for 180 min. Each subject ingested oral glucose (30 g) at 120 min, and the glucose infusion was adjusted to maintain the plasma glucose plateau. Basal insulin and C-peptide concentrations and insulin secretion rates (calculated by the deconvolution method) were significantly greater in obese children compared with lean children (p < 0.001). Similarly, during the first 120 min of the clamp, insulin secretion rates were higher in obese than lean children. After oral glucose, plasma insulin, C-peptide, and insulin secretion rates further increased in all four groups. This incretin effect was 2-fold greater in obese versus lean adolescents (p < 0.001). Circulating plasma GIP concentrations were similar at baseline in all four groups and remained unchanged during the first 120 min of the clamp. After oral glucose, plasma GIP concentrations rose sharply in all groups (p < 0.002). Of note, the rise in GIP was similar in both lean and obese children. In conclusion, under conditions of stable hyperglycemia, the ingestion of a small amount of glucose elicited equivalent GIP responses in both lean and obese children. However, despite similar GIP responses, insulin secretion was markedly augmented in obese adolescents. Thus, in juvenile obesity, excessive alimentary β-cell stimulation may be independent of the increased release of GIP.
Similar content being viewed by others
Main
It is well known that glucose ingestion generates a greater elevation in circulating plasma insulin concentrations as compared with i.v. glucose load (1, 2). This augmentation in insulin secretion is mainly caused by the release of gut hormones that act on β-cells to potentiate glucose-induced insulin secretion (3). This effect, known as the incretin effect, is mainly attributed to the GIP produced by the K-cells of the duodenum and jejunum (4–8) and to the GLP1 (7–36 amide) from the L-cells of the ileum (9–15).
The significance of the incretin effect stems from the central importance of insulin and derangements in insulin metabolism in the pathologic states of obesity and type 2 diabetes. Hyperinsulinemia, the most frequent biochemical alteration of obesity, is a consequence of augmented insulin secretion as well as decreased fractional hepatic extraction of insulin (16). The increased insulin secretion, particularly after meals, has been linked in several animal models of obesity to an overactive entero-insular axis (17, 18). For example, the mutant ob/ob mouse exhibits a generalized hyperplasia of the endocrine cell of the small intestine (17), and both intestinal GIP concentrations and circulating concentrations of the hormone are elevated (19). These abnormalities may be secondary to increase energy intake, rather than primary. Moreover, the ob/ob mouse appears to be particularly sensitive to the insulinotropic effect of GIP (20). Similar results have been found in db/db mice (21), but not in Zucker fatty (fa/fa) rats (22).
It is uncertain, however, whether such rodent obese models can define the role of the entero-insular axis in the disordered β-cell function in human obesity. Although some studies have observed elevated fasting GIP concentrations in obese adult subjects (3) and exaggerated GIP responses to oral glucose or to a liquid meal (23–26), other studies have failed to find an augmentation in nutrient-stimulated GIP responses in adult obese subjects (27, 28). Differences in the glucose or meal size and composition may have contributed to the variability of the GIP responses in these studies. It is noteworthy that the integrity of the GIP response has never been examined in childhood obesity, which is clearly associated with dysregulation of the β-cell function early in its development.
In the current study, we used the hyperglycemic clamp with a small load of oral glucose to study the contribution of GIP to glucose-induced insulin secretion in childhood obesity (2, 29). This technique allows separation of the effects of hyperglycemia and intestinal insulinotropic factors on β-cell response.
GIP stimulates insulin secretion only in the presence of mild to moderate hyperglycemia (29). Indeed, GIP secreted in response to fat ingestion alone does not stimulate insulin secretion, serving as a safeguard against inappropriate insulin secretion and hypoglycemia (22). Because GIP insulinotropic action is glucose-dependent, with greater effects occurring at higher glucose concentrations, we have raised plasma glucose to hyperglycemic concentrations (11 mM) to be able to study its potentiating effects on insulin secretion in lean and obese children.
METHODS
Subjects.
Four groups of subjects were studied, and their clinical and biochemical characteristics are indicated in Table 1. The prepubertal children were clinically Tanner stage I with no biochemical evidence of puberty. The adolescents were matched for Tanner stage and ranged between III and IV. Plasma samples for measurement of estradiol and testosterone were also taken as biochemical markers of pubertal development (Table 1).
As shown in Table 1, weight (in kilograms) and BMI (kilograms per square meter) were significantly greater in the obese subjects. IGF-I was highest in the lean adolescents compared with all other groups (p < 0.01). The obese subjects were recruited from the Yale Pediatric Weight Management Clinic; they all had a BMI >95th percentile specific for age and sex (based on percentile curves for white girls and boys computed from the first National Health and Nutrition Examination Survey 1971–1974) (30). Percentage of ideal weight for height were >150% for the obese children and <120% for the lean children. All subjects were in good health and taking no medications, and none were attempting to restrict caloric intake before study. All subjects were normally active, and none were participating in an organized physical training program. The Human Investigation Committee of the Yale University School of Medicine approved all study protocols. Before participation in the study, the nature, purpose, and requirements of the study were explained thoroughly to all subjects and a parent, and written consent was obtained.
Hyperglycemic clamp and oral glucose: incretin stimulationtest.
Subjects were studied in the Children's Clinical Research Center at the Yale University School of Medicine at 0800 after a 12-h overnight fast. Two i.v. catheters were inserted before the hyperglycemic clamp study: one in an antecubital vein for administration of glucose and the other into a vein of the hand or distal forearm of the contralateral arm for blood sampling. The hand chosen for blood withdrawal was placed in a heated box at approximately 65°C to arterialize blood. During the study, after the collection of baseline blood samples to measure fasting glucose, insulin, C-peptide, and GIP concentrations, the plasma glucose concentration was acutely raised to 11 mM by infusing 20% dextrose. Plasma glucose was measured at 5-min intervals and held constant at this hyperglycemic concentration for 180 min by appropriate adjustment of a variable-rate 20% glucose infusion. Blood samples were also obtained at 2, 4, 6, 8, and 10 min and then every 10 min thereafter for hormone measurements. At 120 min, subjects ingested, in <3 min, 90 mL of a glucose solution containing 30 g of glucose (General Medical Corporation, Richmond, VA). Blood samples were drawn every 5 min, and glucose concentrations were measured. The rate of i.v. infusion of exogenous glucose was adjusted to maintain plasma glucose at the same hyperglycemic plateau despite the ingestion of oral glucose.
Analyses.
Plasma glucose concentrations were measured by the glucose oxidase method with a Beckman glucose analyzer (Beckman Instruments, Brea, CA). Plasma insulin and C-peptide were measured by a double-antibody RIA in the Yale General Clinical Research Center Core Laboratory. Plasma GIP was measured by Dr. Ray Pederson at the University of British Columbia by RIA as described by Kuzio et al. (6). This assay does not show reactivity to gastrin, glucagon, secretin, cholecystokinin, pancreozymin, motilin, or VIP and has no cross-reactivity with GLP1. The intra- and interassay variability was 6.2% and 7.8%, respectively.
Determination of ISR.
ISRs were calculated by deconvolution of plasma C-peptide concentrations using a two-compartment model of C-peptide kinetics as previously described (31). We used standard kinetic parameters describing C-peptide clearance based on 200 individual decay curves of biosynthetic C-peptide in normal, obese, and diabetic adults (32). These parameters account for differences on the basis of age, sex, and body surface area. The area under the insulin secretion rates and insulin concentrations were calculated from 0 to 120 min and from 120 to 180 min of the hyperglycemic clamp, which represents both the glucose plus the incretin effects. The ratio between these two areas provided an indirect measure of endogenous insulin clearance before and after the oral glucose load (32, 33).
Statistical analysis.
All values are presented as mean ± SEM. Statistical analyses were performed using two-way ANOVA with a repeated measure design and by two-tailed paired t test. Pearson correlation coefficients were calculated to assess the degree of association between variables. AUCs were determined using the trapezoidal rule between 0 and 120 min and between 120 to 180 min of the study. Differences were regarded as statistically significant if the corresponding p ≤ 0.05. All statistical analyses were performed using the SAS computer analysis program (version 6; SAS Institute, Cary, NC).
RESULTS
Basal and stimulated insulin and C-peptide concentrations andISRs.
Figure 1 illustrates the plasma glucose, insulin, and C-peptide concentrations during the hyperglycemic clamp with oral glucose in the lean and obese preadolescents and adolescents. In all groups, the plasma glucose was raised and kept at 11 mM by a variable infusion of 20% glucose. This hyperglycemic plateau was maintained even after the ingestion of 30 g of oral glucose at 120 min of the clamp.
Basal plasma insulin and C-peptide concentrations were greater in the obese groups than in the lean children (p < 0.01). During the first 120 min of the study, the plasma insulin concentrations displayed the characteristic biphasic response, which was preserved in the obese group, albeit at a much higher concentration. After the ingestion of the small glucose load, plasma insulin concentrations increased further in all four groups. The magnitude of the insulin response to oral glucose was greater only in the obese adolescents compared with lean children (p < 0.001). In the obese preadolescents, the effect was not significantly different from the lean children. Circulating plasma C-peptide concentrations also displayed a similar pattern of response before and after the oral glucose load in both lean and obese children.
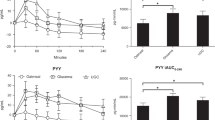
Basal absolute ISRs were 2- to 3-fold higher in obese preadolescent and adolescents (116 ± 16 and 344 ± 74 pmol/mL, respectively) than lean preadolescents and adolescents (65 ± 18 and 102 ± 9 pmol/mL, respectively;p < 0.001; Fig. 2). In both lean and obese preadolescent and adolescent children, basal ISRs were significantly correlated with basal insulin concentrations (r = 0.46, p < 0.03;r = 0.67, p < 0.001; lean and obese, respectively). During the hyperglycemic clamp, ISRs increased promptly from basal levels to reach peak values at 4 min that were markedly higher in the obese groups than both lean groups (p < 0.0001). After the early peak of insulin secretion there was a fall, with a subsequent gradual rise in ISRs in all four groups. However, these rates were greater in the obese children throughout the first 120 min of the clamp. The AUCs for insulin secretion were also significantly greater in obese children during the first 120 min of the clamp (Fig. 3). Ingestion of oral glucose further stimulated significantly ISRs in all four groups. As shown in Figure 3, after the glucose load, the AUCs for the ISR were significantly only in obese adolescent children compared with lean adolescent children (p < 0.001).
To determine whether the clearance of insulin was different between lean and obese children, we calculated the ratio between the AUC of the insulin secretion and the AUC of the peripheral insulin concentration before and after the oral glucose load. This ratio, which provides an indirect measure of endogenous insulin clearance, was slightly but not significantly lower in obese preadolescents and adolescents (10.7 ± 1.1 and 10.4 ± 1.1 mL/min, respectively) than in lean preadolescents and adolescents (11.5 ± 0.7 and 12.9 ± 0.5 mL/min, respectively). After the ingestion of the small glucose load, the clearance of insulin fell significantly in both obese (6.1 ± 0.8 and 6.8 ± 0.8 mL/min, respectively) and lean children (6.6 ± 0.8 and 7.9 ± 0.7 mL/min, respectively) children, reaching values that were virtually identical in all four groups.
GIP Response.
Circulating plasma GIP concentrations were similar at baseline in obese and lean children, and remained unchanged during the first 120 min of the hyperglycemic clamp (Fig. 4). In contrast, in all four groups, plasma GIP concentrations rose sharply after the ingestion of oral glucose (p < 0.02). Of note, the rise in GIP was similar in both lean and obese children.
DISCUSSION
In the present study, we have used the hyperglycemic clamp technique with ingestion of oral glucose to evaluate whether increased GIP response contributes to the hyperinsulinemia of juvenile obesity. The hyperglycemic clamp allowed us to maintain a stable hyperglycemic plateau in all subjects, even after the ingestion of oral glucose. By keeping the hyperglycemic stimulus identical in lean and obese children, we were able to isolate and quantitate the incremental effect of gut factors on glucose-stimulated insulin responses. We found that the ingestion of a small glucose load caused a further stimulation of insulin secretion, which was significantly greater in obese adolescents compared with lean controls. Of particular importance, this alimentary augmentation in insulin secretion occurred despite equivalent GIP responses to the oral glucose loads in lean and obese children. This excessive β-cell stimulation in relation to alimentation may be caused by 1) an intrinsic property of the β-cells of obese subjects, 2) an increased sensitivity to the biologic effect of GIP, or 3) a neural dysregulation of insulin secretion. The ingestion of only 30 g of glucose was followed by a marked rise in insulin secretion, which was paralleled by a rise in plasma GIP concentrations. We excluded a time-dependent effect of insulin secretion because in a previous study, we found a clear-cut difference between the hyperglycemic clamp plus oral glucose and the control experiment (hyperglycemic clamp with no oral glucose) (34). Using a similar paradigm but a greater oral glucose load, Elahi et al. (35) reported a greater GIP response in obese adults. Others (27, 28), however, reported no significant differences in postprandial GIP responses to mixed meals of varying size in obese and lean subjects, despite a greater insulin secretion in the obese subjects.
GIP and GLP1 insulinotropic effects are glucose dependent, with greater effects occurring at higher glucose concentrations (29). Hence, the hyperglycemic condition used in the present study has clearly allowed us to examine the potentiating effects of this gut factor on insulin secretion. At euglycemic concentrations, GIP has no effect on the β cell, and it stimulates insulin secretion when the plasma glucose concentration exceeds 110 mg/dL (6 mM) (22). In fact, it has been suggested that GIP should be renamed glucose-dependent insulinotropic polypeptide (29). Although under the conditions used here glucose was clamped at higher concentrations, we have evidence that some obese children do exhibit mild postprandial hyperglycemia, even though their carbohydrate tolerance is not impaired (Sonia Caprio, personal communication).
It is clear that GIP alone cannot account for the full incretin effect normally associated with a mixed meal and that additional effects are caused by the insulinotropic effects of GLP1 (7–36) (29). It should be emphasized that, on a molar basis, the increment in GIP after oral glucose load (75 g) or a standard breakfast is nearly 4 times greater than that of GLP1 (36). From the higher plasma concentrations of GIP in the basal and postprandial states, it has been suggested that physiologically GIP is the quantitatively more important incretin hormone (37).
The significant rise in GIP concentrations after only the ingestion of 30 g of glucose (120 cal) undoubtedly mediated the acute stimulation in insulin secretion seen in both lean and obese children. We, however, cannot exclude that other incretins, particularly GLP1, were also released under the present conditions, thereby synergizing with GIP to increase insulin secretion. This possibility remains to be explored.
In the present study, we have used a two-compartment model to estimate ISRs from peripheral C-peptide concentrations (31). Using this quantitative analysis, we found increased basal insulin concentrations and first- and second-phase plasma insulin responses in lean pubertal versus lean prepubertal children that could be accounted for by increased ISRs. Moreover, the ISRs under these conditions were greatly exaggerated by the presence and severity of obesity. Indeed, a highly significant linear relationship was found between basal (r = 0.56, p < 0.001) and stimulated (r = 0.62, p < 0.003) insulin secretion and BMI in obese children. Our data suggest that the hyperinsulinemia observed in obese children is not accounted for by reduced fractional clearance rate of insulin. By calculating the ratio between the AUC for the ISR and the AUC for the peripheral insulin concentration, we obtained an overall estimate of endogenous insulin clearance. The units of this ratio are milliliters per minute, and inasmuch as it relates insulin secretion to peripheral insulin concentrations, it is an indirect measure of endogenous clearance as described by Polonsky et al. (16). In contrast to the data reported in obese adults (16), the insulin clearance was similar in lean and obese children, and no relation was found between insulin clearance and BMI. Our study suggests that increased insulin secretion is the most important factor in the pathogenesis of hyperinsulinemia of childhood obesity, whereas insulin clearance seems to be less affected. It should be noted that the C-peptide kinetic factors used for calculation of ISRs were based on data in adult populations, and data based on C-peptide decay curves from a pediatric population would be ideal. To our knowledge, however, no such data exist. Biosynthetic C-peptide is currently unavailable to perform these studies.
In conclusion, we found that under the condition of stable hyperglycemia, ingestion of a small amount of glucose elicited equivalent GIP responses in both lean and obese adolescents. However, despite similar GIP concentrations, insulin secretion was markedly enhanced in obese adolescents. Thus, in juvenile obesity, excessive alimentary β-cell stimulation may be independent of the increased release of gut hormones such as GIP.
Abbreviations
- GIP:
-
gastric inhibitory peptide
- ISR:
-
insulin secretion rate
- GLP1:
-
glucagon-like peptide-1
- AUC:
-
area under the curve
- BMI:
-
body mass index
References
Elrick H, Stimmler L, Hlad CJ, Arai Y 1964 Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol 24: 1076–1079.
Andersen D, Elahi D, Brown JC, Tobin JD, Andres R 1978 Oral glucose augmentation of insulin secretion. J Clin Invest 62: 152–161.
Ebert R, Creutzfelt N 1987 Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev 3: 1–26.
Brown JC, Mutt V, Pederson RA 1970 Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol Lond 209: 57–64.
Beck B 1989 Gastric inhibitory polypeptide: a gut hormone with anabolic functions. J Molec Endocrinol 2: 169–174.
Kuzio M, Dryburgh JR, Malloy KM, Brown JC 1974 Radioimmunoassay for gastric inhibitory polypeptide. Gastroenterology 66: 357–364.
Brown JC, Otte SC 1978 Gastrointestinal hormones and the control of insulin secretion. Diabetes 27: 782–789.
Elahi D, Andersen DK, Brown JC, Debas HT, Hershcopf RJ, Raizes GS, Tobin JD, Andres R 1979 Pancreatic alpha- and beta-cell responses to GIP infusion in normal man. Am J Physiol 237: E185–E191.
Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L 1986 Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 261: 11880–11889.
Holst JJ, Orskov C, Nielsen OV, Schwartz TW 1987 Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 211: 169–174.
Mojsov S, Weir GC, Habener JF 1987 Insulinotropin: glucagon-like peptide 1 (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 79: 616–619.
Thorens B, Weber G 1993 Glucagon-like peptide-1 and the control of insulin secretion in the normal state and in NIDDM. Diabetes 42: 1219–1225.
Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF 1987 Glucagon-like peptide-1 stimulates insulin gene expression and increases cyclic AMP levels in rat islet cell line. Proc Natl Acad Sci USA 84: 3434–3438.
Thorens B 1992 Expression cloning of the pancreatic β-cell receptor for the glucoincretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89: 8641–8645.
Weir GC, Mojsov S, Hendrick GK, Habener JF 1989 Glucagon-like peptide-1 (7–37) actions on endocrine pancreas. Diabetes 38: 338–342.
Polonsky KS, Given BD, Hirsh L, Shapiro ET, Tillil H, Beebe C, Galloway JA, Frank BH, Karrison T, Van Cauter E 1988 Quantitative study on insulin secretion and clearance in normal and obese subjects. J Clin Invest 81: 435–441.
Pollack MJ, Pearse A, Grimelius L, Marks V 1975 Gastrointestinal apulosis in obese hyperglycemic mice. Virchows Arch B Cell Pathol 19: 135–150.
Chan CB, Peberson RA, Buchan AMJ, Tubesing KB, Grown J 1984 Gastric inhibitory polypeptide (GIP) and insulin release in the obese Zucker rat. Diabetes 33: 536–542.
Best LC, Atkins T, Bailey W, Flatt OR, Newton DF, Matty AJ 1977 Increased activity of the enteroinsulin axis in obese hyperglycemic mice (ob/ob). J Endocrinol 72: 44–47.
Flatt PR, Bailey CJ, Kwasowski P, Swanston-Flatt SK, Marks VI 1983 Abnormalities of GIP in spontaneous syndromes of obesity and diabetes in mice. Diabetes 32: 433–435.
Bailey CJ, Flatt PR 1988 The entero-insulin axis in models of hyperinsulinemic and hypoinsulinemic diabetes. In: Shafrir E, Renold AE (eds) Frontiers in Diabetes Research: Lessons from Animal Diabetes, Vol 2. John Libbey and Co, London, 277–224.
Morgan LM 1992 Insulin secretion and the entero insulinar axis. In: Flatt PR (ed). Portland Press, London and Chapel Hill, 1–22.
Creutzfeldt W, Ebert R, Willms B, Frerichs H, Brown JC 1978 Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia 14: 15–24.
Creutzfeldt W, Ebert R 1985 New developments in the incretin concept. Diabetologia 28: 565–573.
Dupre J, Ross SA, Watson D, Brown JC 1973 Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 37: 826–828.
Ebert R, Frerichs H, Creutzfeldt W 1979 Impaired feedback control of fat induced gastric inhibitory polypeptide (GIP) secretion by insulin in obesity and glucose intolerance. Eur J Clin Invest 9: 129–135.
Lauritsen KB, Christensen KC, Stokholm KH 1980 Gastric inhibitory polypeptide (GIP) release and incretin effect after oral glucose in obesity and after jejunoileal bypass. Scand J Gastroenterol 15: 489–495.
Service FJ, Rizza RA, Westland RE, Hall LD, Gerich J, Go VLW 1984 Gastric inhibitory polypeptide in obesity and diabetes mellitus. J Clin Endocrinol Metab 58: 1133–1136.
Fehman HC, Göke R, Göke B 1995 Cell and molecular biology of the incretin hormones glucagon-like peptide I and glucose-dependent insulin releasing polypeptide. Endocr Rev 16: 390–410.
Hammer LD, Kraemer HC, Wilson DM, Ritter RL, Dornbusch SM 1991 Standardized percentile curves of body mass index for children and adolescents. Am J Dis Child 145: 259–263.
Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B 1986 Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest 77: 98–105.
Eaton RP, Allen RD, Schade DS, Erickson KM, Standefer J 1980 Prehepatic insulin production in man: peripheral analysis using connecting peptide behavior. J Clin Endocrinol Metab 51: 520–528.
Van Cauter E, Mestrez F, Sturis J, Polonsky KS 1992 Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41: 368–377.
Limb C, Tamborlane W, Sherwin RS, Pederson R, Caprio S 1997 Acute incretin response to oral glucose is associated with stimulation of gastric inhibitory polypeptide, not glucagon-like peptide in young subjects. Pediatr Res 41: 364–367.
Elahi D, Andersen DK, Muller DC, Tobin JD, Brown JC, Andres R 1984 The enteric enhancement of glucose-stimulated insulin release. Diabetes 33: 950–957.
Kreymann B, Williams G, Ghatei MA, Bloom SR 1987 Glucagon-like peptide 1(7–36): a physiological incretin in man. Lancet 2: 1300–1304.
Jua X, Brown JC, Pederson RA, McIntosh CH 1995 Effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide I (7–36) on insulin secretion. Am J Physiol 268: E645–E651.
Acknowledgements
The authors are particularly grateful to all the children for participating in the study. We thank the nursing staff for the excellent care given to our subjects during these studies, the staff of the Core Laboratory of the Clinical Center for their technical assistance, and Nancy Canetti for the superb preparation of the manuscript.
Author information
Authors and Affiliations
Additional information
Dr. Rubina A. Heptulla is a recipient of a postdoctoral award from the Juvenile Diabetes Foundation. This work was supported by grants from the National Institutes of Health (DK 20495, HD 30671, RR 06022, HD 28016, and RR 00125).
Rights and permissions
About this article
Cite this article
Heptulla, R., Tamborlane, W., Cavaghan, M. et al. Augmentation of Alimentary Insulin Secretion despite Similar Gastric Inhibitory Peptide (GIP) Responses in Juvenile Obesity. Pediatr Res 47, 628–633 (2000). https://doi.org/10.1203/00006450-200005000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200005000-00012