Abstract
Cyclooxygenases-1 and -2 are the key enzymes in the conversion of arachidonic acid to prostanoids. Cyclooxygenase-2 (COX-2) takes part both in inflammation and in control of cell growth. COX-2 immunohistochemistry was performed on lung tissues from autopsies, with four groups included: fetuses (n = 4, GA = 16.0 to 32.0 wk), preterm infants (n = 10, GA = 23.0 to 29.9 wk), term infants (n = 6, GA = 38.7 to 42.0 wk), and infants with bronchopulmonary dysplasia (BPD) (n = 4, GA = 28.9 to 30.7 wk). COX-2 staining occurred exclusively in the epithelial cells resembling type II pneumocytes in the alveolae, and in ciliated epithelial cells in the bronchi. In fetuses, moderate intensity alveolar staining was seen in 90–100% cells lining the alveolar epithelium. In preterm infants, high intensity alveolar staining was seen in a scattered pattern. In term infants, the alveolar staining was also scattered, but with a lower proportion of positive cells. In BPD no staining appeared in alveolar epithelial cells. The most intense bronchial staining was found in fetuses and the least intense in term infants; staining was also seen in BPD. COX-2 is present in human perinatal lung from the gestational age of 16 wk, in a changing pattern. We suggest that COX-2 may, in addition to participating in inflammation, also play a developmental role in the perinatal lung.
Similar content being viewed by others
Main
Cyclooxygenase, also known as prostaglandin H synthase and prostaglandin endoperoxide synthase, is the key enzyme in the conversion of arachidonic acid to prostanoids and other eicosanoids. Two distinct cyclooxygenases exist, often coexpressed in the same cell (1–3). Cyclooxygenase-1, a constitutive enzyme produced constantly in most tissue types, is probably responsible for the production of prostanoids under physiologic conditions, whereas, although cyclooxygenase-2 (COX-2) is undetectable in most tissues, its expression can be induced rapidly in various cell types (4–6). A wide range of stimuli may induce COX-2 expression, including proinflammatory agents, growth factors, and oncogenes, but antiinflammatory cytokines and glucocorticoids decrease it (7). COX-2 expression is increased in several pathophysiological conditions, including acute and chronic inflammation and carcinogenesis (6, 8, 9).
In the developing lung, cyclooxygenase-dependent pathways are involved in a large array of physiologic processes. Among these are regulation of lung vascular tone, vascular and interstitial tissue remodeling, regulation of capillary endothelial and alveolar epithelial permeability, and surfactant homeostasis (10–14). In the ovine lung, COX-2 expression increases during the perinatal period (15). Because products of cyclooxygenase-dependent pathways may play a role in the physiology and pathophysiology of the developing lung, we wanted to examine by immunohistochemistry whether COX-2 is present in the lungs of human infants during the perinatal period, as well as in the lungs of infants with bronchopulmonary dysplasia (BPD).
METHODS
Patients.
With the approval of the Ethics Committee of the Hospital for Children and Adolescents, University Central Hospital, Helsinki, a total of 20 infants and 4 fetuses who died between March 1987 and January 1999 in the University Central Hospital, Helsinki, Finland, were included. Infants with congenital anomalies (term infants with other than cardiac anomalies) or with pneumonia at the time of death had been excluded. Autopsies were performed within 2 d after death. Patients were divided into four groups.
Group A: Fetuses (n = 4, GA = 16.0 to 32.0 wk) Three of these four pregnancies were terminated due to fetal anomalies, but autopsies showed all to have macroscopically and microscopically normal lungs. Group B: Preterm infants (n = 10, GA = 23.0 to 29.9 wk) who died of immaturity and respiratory distress syndrome (RDS). Autopsies showed hyaline membranes in the lungs of all these patients. Group C: Term infants (n = 6, GA = 38.7 to 42.0 wk) who died of congenital cardiac anomalies. On autopsy, pulmonary edema was found in three infants, but lung histology was otherwise normal. Group D: Infants with BPD (n = 4, GA = 28.9 to 30.7 wk) who died from chronic lung disease, and had been intubated for almost all of their lives due to respiratory insufficiency. On autopsy all had significant changes in their lungs typical for BPD, and one infant also had emphysema (Table 1).
COX-2 immunohistochemistry.
The lung samples obtained from autopsies were fixed in 10% neutral buffered formalin, embedded in paraffin blocks and kept in dry storage at room temperature. After cutting from blocks, the slides were used within 2 wk. Tissue samples were sectioned (4–5 μm), deparaffinized, and microwaved for 4–5 min in 0.01 M Na-citrate buffer (pH 6.0). The slides were first immersed in 1.6% hydrogen peroxide in methanol for 30 min and then in blocking solution (0.01 M Tris, 0.1 M MgCl2, 0.5% Tween, 1% BSA, and 5% normal goat serum) for 1 h to block endogenous peroxidase activity and unspecific binding sites, respectively. Immunostaining was performed with a rabbit polyclonal IgG against a mouse COX-2 peptide (Cayman Chemical Co., Ann Arbor, MI) in a dilution of 1:600 in the blocking solution at 4°C overnight. The sections were thereafter treated with biotinylated secondary antibodies at a dilution of 1:250 (Vectastain ABC kit, Vector Laboratories, Burlingame, CA), and antibody-binding sites were finally visualized by avidin-biotin peroxidase complex solution (ABComplex, Vectastain, Vector Laboratories) and 3,3′-diaminobenzidine. As a control, nonspecific rabbit IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA) at a concentration corresponding to that of the COX-2 antibody (1:200) was used to stain all sections by an identical protocol (9). All samples were analyzed by H.W. by microscopy. The pathologist was blinded in respect to the samples. The intensity of staining was quantified on a scale from 1 to 5, with 1 indicating barely detectable staining and 5 indicating maximal staining. Intensity and frequency of the staining were judged separately.
Statistical methods.
Patient data are given as mean and range, and results as mean ± SEM. Comparisons between groups were performed with one-way ANOVA. The Bonferroni correction was used for the post hoc comparisons. Simple regression analysis was used for continuous variables. p-Values less than 0.05 were considered statistically significant. All calculations were performed with StatView 4.1.
RESULTS
No differences existed between groups in regard to time from death to autopsy. The death of the fetuses was more recent, but the chronological distributions between other groups did not differ. COX-2 was detected in all specimens. Staining was found exclusively in the epithelial cells of the alveolae and bronchi, with none in the interstitium, inflammatory cells, or endothelium. In all groups, bronchial staining was present, localized in ciliated epithelial cells. Where present, alveolar staining was seen in cells resembling type II pneumocytes and in cuboidal cells in fetuses.
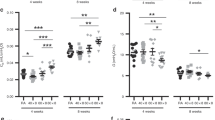
Alveolar staining in the fetuses was seen in most of the cells lining the alveolar epithelium. Intensity of the staining was moderate in all specimens (Fig. 1a, Table 2). In preterm infants, the alveolar staining was most intense in areas with hyaline membranes. The intensity of the staining was high, appearing in cells resembling type II pneumocytes (Fig. 1b, Table 2). In term infants, the pattern of alveolar staining was similar to that seen in preterm infants, but with a lower proportion of positive cells (Fig 1c, Table 2). In BPD no staining appeared in alveolar epithelial cells (Fig. 1d, Table 2). In three cases with BPD and with clinically and microscopically verified pneumonic infection at the time of death, alveolar staining showed a pattern similar to that in preterm infants (data not shown). In some samples, an infiltration of granulocytes was apparent, and in these cells an occasional positivity was observable.
The most intense bronchial staining was encountered in fetuses and the least intense in term infants. Significant bronchial staining was present also in BPD (Table 2).
The staining of the samples was quantified by calculation of a staining index [intensity of the staining (1 to 4) × percentage of the cells stained]. The alveolar and bronchial staining indices correlated negatively with gestational age (R = −0.48, p = 0.042; −0.45, p = 0.031, respectively), and with birth weight (R = −0.53, p = 0.025; R = −0.50, p = 0.015, respectively) (Fig. 2).
DISCUSSION
We show here that COX-2 is present in the human perinatal lung from the gestational age of 16 wk to term neonates. The pattern of the presence of COX-2 in the lungs changes during the perinatal period. COX-2 is also seen in the lungs of infants with bronchopulmonary dysplasia, but in a pattern different from that in infants during the perinatal period.
In ovine lungs under physiologic conditions, COX-2 expression has been shown to increase from the fetal to the newborn period (15). In the developing lung, cyclooxygenase-dependent pathways are involved in several physiologic processes, including a potential constitutive role for COX-2 in maintaining fetal ductus arteriosus patency in late-gestation lambs (16). COX-2 may play a role in the regulation of mitogenesis, by increasing intestinal epithelial cell adhesion to extracellular matrix and by inhibiting apoptosis (17, 18). Moreover, COX-2 has been shown to regulate cancer-induced angiogenesis, possibly by modulating production of angiogenic factors by colon cancer cells (19). We found COX-2 exclusively in epithelial cells resembling type-II cells in the alveolae, and in the ciliated epithelial cells lining the bronchi. In contrast, no staining was seen in interstitial cells, in inflammatory cells, or endothelial cells. This finding is in line with type-II pneumocytes' being capable of producing several types of factors that have mitogenic properties (20). In the lungs of human adults, the presence of COX-2 is associated mainly with severe pathologic conditions (9). In our study, fetal lungs showed the highest staining, and a negative correlation existed between staining and gestational age. These data suggest a physiologic function for COX-2 in the developing human lung, a function possibly controlled by alveolar type-II pneumocytes.
COX-2 may be induced by several types of stimuli, including inflammatory mediators and hyperoxia (7, 21). In preterm infants, significant amounts of 6-keto-PGF1alfa and TXB2 have been detected in tracheoalveolar lavage (Lassus, et al. unpublished observations). It is possible that the COX-2 and prostanoids seen in preterm infants with respiratory distress is due at least in part to an inflammatory response under hyperoxia. In patients with BPD, COX-2 was detected in the bronchial, but not in the alveolar epithelium. In contrast, in BPD patients with pneumonia, COX-2 was detected also in the alveolar epithelium. In rats, COX-2 is expressed constitutively in the epithelium of large bronchi but not in alveolae (22). The presence of COX-2 in alveolae in BPD patients with pneumonia may be a result of COX-2 stimulation by infection. In animal lungs, COX-2 has been shown to induce bronchoconstriction in vivo (23). Therefore, COX-2 seen in the bronchial epithelium may participate in the bronchial hyperreactivity associated with BPD (24). One preterm infant had received antenatal steroids, and three infants who developed BPD had received postnatal dexamethasone. It is expected that glucocorticoids will affect COX-2, but this factor was impossible to evaluate in the present study (7).
In conclusion, this study shows that COX-2 is present in human perinatal lung in a changing pattern during the perinatal period. We suggest that COX-2 may, in addition to participating in inflammation, also have a developmental role in the perinatal lung.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- COX-2:
-
cyclooxygenase-2
- GA:
-
gestational age
- RDS:
-
respiratory distress syndrome
References
Maier JA, Hla T, Maciag T 1990 Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem 265: 10805–10808.
Hla T, Neilson K 1992 Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA 89: 7384–7388.
O'Neill GP, Ford-Hutchinson AW 1993 Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett 330: 156–160.
Mitchell JA, Belvisi MG, Akarasereenont P, Robbins RA, Kwon OJ, Croxtall JJ, Barnes PJ, Vane JR 1994 Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br J Pharmacol 113: 1008–1014.
DeWitt DL, Meade EA 1993 Serum and glucocorticoid regulation of gene transcription and expression of the prostaglandin H synthase-1 and prostaglandin H synthase-2 isozymes. Arch Biochem Biophys 306: 94–102.
Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA 1994 Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA 91: 2046–2050.
Vane JR, Bakhle YS, Botting RM 1998 Cyclooxygenases 1 and 2. Ann Rev Pharmacol Toxicol 38: 97–120.
Hla T, Ristimaki A, Appleby S, Barriocanal JG 1993 Cyclooxygenase gene expression in inflammation and angiogenesis. Ann NY Acad Sci 696: 197–204.
Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimäki A 1998 Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 58: 4997–5001.
Acarregui MJ, Snyder JM, Mitchell MD, Mendelson CR 1990 Prostaglandins regulate surfactant protein A (SP-A) gene expression in human fetal lung in vitro. Endocrinology 127: 1105–1113.
Holtzman MJ 1991 Arachidonic acid metabolism: implications of biological chemistry for lung function and disease. Ann Rev Respir Dis 143: 188–203.
Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE 1992 An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 327: 70–75.
Suttorp N, Weber U, Welsch T, Schudt C 1993 Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest 91: 1421–1428.
Arias-Diaz J, Vara E, Garcia C, Balibrea JL 1994 Tumor necrosis factor-alpha-induced inhibition of phosphatidylcholine synthesis by human type II pneumocytes is partially mediated by prostaglandins. J Clin Invest 94: 244–250.
Brannon TS, MacRitchie AN, Jaramillo MA, Sherman TS, Yuhanna IS, Margraf LR, Shaul PW 1998 Ontogeny of cyclooxygenase-1 and cyclooxygenase-2 gene expression in ovine lung. Am J Physiol 2:L 66: 71
Takahashi Y, Maziasz T, Paulson S, Isakson P, Heymann MA, Chemtob S, Clyman RI 1999 Cyclooxygenase-2 (COX-2) plays a significant role in regulating the fetal ductus arteriosus in vivo and in vitro. Pediatr Res 45: 70A
DuBois RN, Awad J, Morrow J, Roberts LJ II, Bisho PR 1994 Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alfa and phorbol ester. J Clin Invest 93: 493–498.
Tsujii M, DuBois R 1995 Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 83: 493–501.
Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN 1998 Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93: 705–716.
Hermans C, Bernard A 1999 Lung epithelium-specific proteins. Am J Respir Crit Care Med 159: 646–678.
Adawi A, Zhang Y, Baggs R, Finkelstein J, Phipps RP 1998 Disruption of the CD40-CD40 ligand system prevents an oxygen-induced respiratory distress syndrome. Am J Pathol 152: 651–657.
Ermert L, Ermert M, Goppelt-Struebe M, Walmrath D, Grimminger F, Steudel W, Ghofrani HA, Homberger C, Duncker H-R, Seeger W 1998 Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am J Respir Cell Mol Biol 18: 479–488.
Uhlig S, Nüsing R, von Bethmann A, Featherstone RL, Klein T, Brasch F, Müller K-M, Ullrich V, Wendel A 1996 Cyclooxygenase-2-dependent bronchoconstriction in perfused rat lungs exposed to endotoxin. Mol Med 2: 373–383.
Bancalari E 1988 Pathogenesis of bronchopulmonary dysplasia: An overview. In: Bancalari E, Stocker JT (eds) Bronchopulmonary Dysplasia. Hemisphere, New York, 3–15.
Acknowledgements
The authors thank Tuula Stjernvall for excellent technical assistance, and Carolyn Norris, Ph.D., for linguistic revision of the manuscript.
Author information
Authors and Affiliations
Additional information
Supported by Finska Läkaresällskapet, the Orion Research Fund, and the Helsinki University Central Hospital Research Fund.
Rights and permissions
About this article
Cite this article
Lassus, P., Wolff, H. & Andersson, S. Cyclooxygenase-2 in Human Perinatal Lung. Pediatr Res 47, 602–605 (2000). https://doi.org/10.1203/00006450-200005000-00008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200005000-00008