Abstract
Total parenteral nutrition is associated with osteopenia in preterm infants. Insufficient calcium and phosphate are likely causes; aluminum contamination is another possible contributing factor as this adversely affects bone formation and mineralization. The study was designed to evaluate changes in biochemical markers of bone turnover in 22 preterm infants receiving total parenteral nutrition in comparison with 19 term infants. We collected urine and serum samples from 22 preterm infants, mean gestational age 29 wk, within 48 h and again at 3 wk of life. We also collected urine samples from 19 term infants, mean gestational age 39 wk, during the first day of life. Bone resorption was assessed by the measurement of urinary pyridinium cross-links by HPLC and ELISA and the N-telopeptide of type I collagen by ELISA. Bone formation was assessed in premature infants by the measurement of serum osteocalcin. The N-telopeptide of type I collagen was higher in the preterm infants compared with term at baseline (p < 0.01). There was no difference between the pyridinium cross-links in the preterm and term infants. All the biochemical markers of bone turnover increased significantly in the preterm infants during the first 3 wk of life, e.g. N-telopeptide was a 153% change from baseline (p < 0.001). Aluminum in the total parenteral nutrition solutions did not cause a decrease in bone formation at the level administered (3-6 µg, 0.1-0.2 µmol·kg-1·d-1).
Similar content being viewed by others
Main
Osteopenia is a common problem in preterm infants. It is possibly related to inadequate calcium and phosphate intake and to aluminum loading from parenteral nutrition(1). Lyon et al.(2) reported the mean intake of calcium and phosphate in preterm infants to be considerably less than the intrauterine accumulation of these minerals and proposed this to be a possible cause of poor bone mineralization. Aluminum, a known contaminant of TPN solutions, has been shown to have a toxic effect on bone formation and mineralization. The sources of such contamination are primarily the salts of calcium and phosphate(3). The effects of aluminum on bone have been evaluated by a combination of static and dynamic bone histomorphometry(4). This technique is not performed in premature infants.
Using static histomorphometry alone, Beyers et al.(5) proposed that increased bone resorption rather than impaired formation resulted in the development of osteopenia in the preterm neonate. Biochemical markers of bone turnover can be used to assess short-term changes, which reflect changes in the whole skeleton, not just a localized area. Most bone resorption markers are based on products of type I collagen degradation: Pyd, which is also found in skin and other connective tissues; Dpd, more specific to bone; and NTx(6–8). Osteocalcin is a commonly used marker for bone formation. It is the most abundant noncollagenous protein found in bone. Its biosynthesis, which is vitamin K-dependent, occurs in the osteoblast. Any osteocalcin that is not incorporated into the bone is released into the circulation(8). Levels of osteocalcin are elevated in conditions of rapid bone turnover(9,10)
The aim of this study was to test the hypothesis that aluminum can interfere with bone formation at levels of contamination of TPN solutions that delivered 15-30 µg·kg-1·d-1 to premature infants(11) and to determine whether there was a predominance of bone resorption compared with bone formation.
METHODS
Subjects. We recruited 22 consecutive preterm infants (10 males, 12 females), mean age 29 wk gestation, range 25 to 35 wk. The birth weight was 1056 ± 208 g (mean ± SD). We also recruited 19 consecutive full-term infants (9 males, 10 females), mean age 39 wk gestation, range 38 to 41 wk. The mean birth weight was 3232 ± 478 g.
Protocol. All preterm infants were given i.v. glucose infusions starting on the first day of life and were started on TPN therapy between d 2 and 3. Composition of the neonatal TPN solution has been previously published(12) and initially contained 5-10% dextrose and 0.5 g protein per kilogram body weight per day as crystalline amino acids (Aminosyn PF, Abbott Laboratories, North Chicago IL). Dextrose concentration was advanced as tolerated up to 25% and protein was gradually increased to 2.5 g·kg-1·d-1. All children received calcium gluconate, 200-400 mg·kg-1·d-1, and potassium phosphate, 1 mmol·kg-1·d-1, in their TPN solutions, along with standard trace elements and multivitamins. Enteral feedings were usually attempted by approximately d 7 of life with most patients reaching full feedings with Similac Special Care (Ross Laboratories, Columbus, OH) by 3 wk of age, at which time TPN treatment was discontinued and no further parenteral aluminum was administered to the infant. For the preterm infants, serum and spot urine samples were collected in the afternoon between 24 and 48 h of life (baseline collection) and at approximately 3 wk [2.6 ± 0.3 wk (±SEM)] at cessation of TPN.
Term infants were started on either human milk or a standard cow's milk-based infant formula, and discharged at 24 to 36 h after birth. The term infants had a spot urine sample collected on the afternoon of the first day of life. Urine collection bags were used to obtain the urine sample and no preservatives were added.
The protocol was approved by the Institutional Review Board of the University of Texas Medical Branch at Galveston, TX. Informed consent was obtained from the subjects' parents.
Measurements. Urinary cross-links of type I collagen, Pyd and Dpd, were measured by HPLC using the method of Colwell et al.(12). HPLC was performed on urine that had been hydrolyzed with acid to measure total cross-links, TPyd and TDpd, intra-assay CV 4.2% and 7.9%, respectively. Urine hydrolysis is not necessary for the measurement of FPyd and FDpd, intra-assay CV 6.2% and 9.6%, respectively. iFDpd was measured in urine by ELISA, Pyrilinks D (Metra Biosystems Inc, Palo Alto, CA)(13). Results were expressed as a ratio to Cr. The intra-assay CV was 6.8% and the detection limit 3 nmol/L. Urinary cross-linked NTx was measured by ELISA, Osteomark (Ostex International Inc, Seattle, WA)(14). Samples were diluted 1:10 with deionized water. Results were expressed as nanomolar bone collagen equivalents (nmol BCE) per millimole Cr. The intra-assay CV was 3.9% and the lower limit of detection was 2.0 nmol BCE. Serum levels of osteocalcin were measured by radioimmunoassay (Nichols Institute, San Juan Capistrano, CA). The intra-assay CV was 4.7% and the detection limit was 0.3 mg/L. As intact osteocalcin cannot be measured in hemolyzed serum samples, it was only possible to measure osteocalcin for 15 of the premature infants.
Total serum calcium was measured using an Ektachem 400 (Eastman Kodak, Rochester, NY). Serum ionized calcium was measured using an ion-specific electrode (Radiometer America, Westlake, OH). Aluminum was quantified by flameless atomic absorption spectroscopy in the urine and TPN solutions of the preterm infants by previously published methods(15). Urinary Cr was measured using a kinetic Jaffe method. All samples were measured in one analytical batch.
Data analysis. All data distributions were tested using the normal probability plot. A two-sample t test was used to compare preterm and term infants. A paired t test was used to evaluate the change in markers in the preterm infants from baseline to 3 wk. Significance was regarded as p < 0.05 for all statistical tests. Regression analysis was used to look at the effect of aluminum on biochemical markers. Statistical analyses was performed using the Statgraphics Plus software program, version 1.4 (STSC, Inc, Rockville, MD).
RESULTS
All of the preterm infants experienced some morbidity. Diagnoses included congenital syphilis-treated (n = 2), documented sepsis (n = 2), respiratory distress (n = 5), bronchopulmonary dysplasia (n = 5), apnea of prematurity (n = 3), retinopathy of prematurity (n = 3), jaundice or cholestasis (n = 4), pneumothorax (n = 2), congenital heart disease (patent ductus arteriosus, ventricular septal defect, and complex disease; n = 7), and cleft palate and vestigial tail (n = 1). None of these underlying conditions have been clearly identified as affecting bone metabolism acutely. Only one of the children in the study received furosemide, and that was one dose at a time after the final urine collection. None received medications known to adversely affect bone.
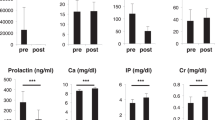
The NTx was elevated in the preterm infants compared with the term infants at baseline. However, there was no significant difference in the pyridinium cross-links measured by HPLC or ELISA in the two groups (Table 1). The absolute values for the bone resorption markers and formation marker (osteocalcin) at baseline and at 3 wk of life are shown in Table 1, along with the baseline values in the term infants. The ratio of Pyd to Dpd, both free and total was not significantly different in the term and preterm infants. The urinary ratio of Pyd to Dpd for total cross-links was 6.6:1 for the preterm infants and 6.3:1 for the term infants; the free ratio was 7.0:1 in the preterm infants and 6.7:1 in the term infants. The percentage of cross-links in the free form was similar for both groups, 35% of Pyd and 33% for Dpd.
The preterm infants showed a postnatal increase in biochemical markers of bone turnover during the first 3 wk of life (Table 1). The largest increase in the bone resorption markers was NTx with a mean change from baseline of 153%, p < 0.001 (95% CI, 88 to 218%). Pyd cross-links measured by HPLC increased, with a mean change in TPyd of 89%, p < 0.01 (95% CI, 33 to 144%); TDpd 74%, p < 0.01 (95% CI, 22 to 126%); and FDpd 63%, p < 0.01 (95% CI, 18 to 108%). The iFDpd showed a similar increase to the FDpd, with a mean increase of 61%, p < 0.05 (95% CI, 15 to 108%). There was a 36% increase in FPyd, p < 0.05 (95% CI, 3 to 69%). The bone formation marker osteocalcin increased by a mean of 361%, p < 0.01 (95% CI, 158 to 564%).
Total serum calcium at baseline in the preterm infants was directly related to gestational age, r = 0.40, n = 22, p < 0.05; range, 6.1 to 9.9 mg/dL (1.52 to 2.47 mmol/L). Aluminum intake in the TPN solutions ranged from 3 to 6 µg·kg-1·d-1 (0.11 to 0.22 µmol·kg-1·d-1). The mean urinary aluminum to Cr ratio was 0.36 ± 0.26 µmol/mmol Cr (n = 17), range, 0.11 to 1.08 µmol/mmol Cr, with normal for term infants being 0.03 ± 0.03 µmol/mmol Cr. Previously published values for aluminum-loaded preterm infants were 0.23 ± 0.19 µmol/mmol Cr(3). The urinary aluminum to Cr ratio was not related to the percentage change in any of the biochemical markers of bone turnover.
DISCUSSION
We have demonstrated a rise in biochemical markers of bone formation (osteocalcin) and resorption (urinary pyridinium cross-links) in premature infants from the first 48 h to the third week of life while receiving TPN treatment with an aluminum load about one third of that reported previously.
The pyridinium cross-links measured by HPLC and ELISA were not significantly elevated in the preterm infants in comparison with the term infants. Tsukahara et al.(16) reported TPyd and TDpd levels to be comparable in 1-mo-old term and preterm infants. The absolute values are comparable with those obtained in this study. The ratio of Pyd to Dpd was not significantly different in the term and preterm infants for both free and total. The ratio for the urinary Pyd:Dpd in premature and term infants was, however, approximately twice the ratio seen in adult urine (3.5:1). The high Pyd in comparison with Dpd could be caused by the cartilaginous nature of neonatal bone. Pyd is abundant in cartilage; this may reflect activity in the growth plate. In the preterm and term infants 33-35% of the Pyd were in the free form. This is less than the percentage in adults with 40-50% of Pyd in the free form. This may be because of inability of the kidney to generate free cross-links owing to immaturity and the high bone turnover state. It has been proposed that a fraction of the FPyd in urine are generated by the kidney from peptide-bound Pyd by cleavage of the oligopeptide amino acids(17). As the neonatal kidney is not mature, this could impair the cleavage of the peptide-bound cross-links, and thus free cross-link generation by the kidneys would be impaired.
The NTx was higher in the preterm infants compared with the term infants. More et al.(18) also found NTx concentration at birth to be higher in preterm infants compared with term infants and reported that gestational age appears to be a major determinant of bone turnover in neonates. It is possible that the placenta produces an enzyme that is involved in NTx degradation, which is not present in the preterm infant. This could result in high NTx levels in preterm urine.
The term infants' urine Cr concentration was higher than that of the preterm infants. Cr excretion is related to muscle mass, and therefore it would be expected for the term infants to have a higher Cr level. The kidneys do not develop their total number of nephrons until 36 wk gestational age, and renal function is not fully developed until the age of 2 y(19), although Cr clearance reaches term values by 34 wk of gestation(3). The low Cr values for the preterm infants are likely to be because of a lower muscle mass.
There was a significant increase in biochemical markers during the first month of life. The largest percentage increase was in osteocalcin. Although osteocalcin biosynthesis is vitamin K dependent, it is unlikely that the dose administered, 1 mg on the first day of life and then daily in the TPN solution as a multivitamin infusate (70 µg for <1 kg, 100 µg for 1 to 3 kg), would contribute significantly to the dramatic increase seen in osteocalcin concentration during the first month of life.
It has been reported that NTx is a more dynamic marker than other markers of bone resorption(20), i.e. it shows a greater percentage change in response to therapy than the pyridinium cross-links. The NTx excretion at 3 wk increased by the largest magnitude in comparison with the other bone resorption markers. markers. The HPLC result for FDpd is in close agreement with the magnitude of change from baseline for the iFDpd. Apone et al.(21) reported that NTx but not FDpd are generated by osteoclastic bone resorption and that FDpd may be generated by breakdown of peptide-bound Pyd in the liver or kidney. This may explain the smaller magnitude of change in the free cross-links.
There is a dramatic postnatal increase in the NTx levels of preterm infants postpartum. A rapid increase in NTx during the first 25 d of life in term infants has also been reported(22). The mean baseline values for the term infants of 1183 nmol/mmol Cr was in close agreement with baseline levels obtained in our study. NTx remained high for 75 d after birth and then began to decrease, stabilizing after 130 d. Lapillone et al.(22) proposed that this may indicate either stabilization of mineral metabolism or reduction in growth rate. It appears that the increase in NTx during the first month of life is similar in preterm and term infants. However, it should be pointed out that although the term infants in our study were healthy, all the preterm infants had some disease. Thus, our study cannot address the question of whether healthy preterm infants would demonstrate changes similar to those we report.
Although there is a possibility that the resorption markers can be found in tissues other than bone, such as cartilage and skin, and therefore not completely reflect postnatal changes in bone, there is little documentation in the literature that sheds light on this issue and therefore this issue must remain speculative at present.
Aluminum intake from TPN contamination at present levels (3-6 µg·kg-1·d-1) and for the duration of the study appears not to affect bone formation as it does at much higher doses in adults(23). Aluminum intake of 15-30 µg·kg-1·d-1 (0.56-1.12 µmol·kg-1·d-1) has been shown to accumulate in bone(24) and has been suggested to impair bone calcium uptake in this population(25). However, the reported TPN aluminum concentration is three- to six-fold higher than in this study.
Abbreviations
- Cr :
-
creatinine
- FDpd :
-
immunoreactive free deoxypyridinoline
- FDpd :
-
free deoxypyridinoline
- FPyd :
-
free pyridinoline
- TDpd :
-
total deoxypyridinoline
- TPyd :
-
total pyridinoline
- Dpd :
-
deoxypyridinoline
- Pyd :
-
pyridinoline
- NTx :
-
N-telopeptide cross-linking region of type I collagen
- TPN :
-
total parenteral nutrition
References
Klein GL, Coburn JW 1991 Parenteral nutrition: effect on bone and mineral homeostasis. Annu Rev Nutr 11: 93–119.
Lyon AJ, Hawkes DJ, Doran M, McIntosh N, Chan F 1989 Bone mineralization in preterm infants measured by dual energy radiographic densitometry. Arch Dis Child 64: 919–923.
Sedman AB, Klein GL, Merritt RJ, Miller NL, Weber KO, Gill WL, Anand H, Alfrey AC 1985 Evidence of aluminum loading in infants receiving intravenous therapy. N Engl J Med 312: 1337–1343.
Klein GL, Alfrey AC, Miller NL, Sherrard DJ, Hazlet TK, Ament ME, Coburn JW 1985 Aluminum loading during total parenteral nutrition. Am J Clin Nutr 35: 1425–1429.
Beyers N, Esser M, Alheit B, Roodt M, Wiggs B, Hough SF 1994 Static bone histomorphometry in preterm and term babies. Bone 15: 1–4.
Robins SP 1995 Collagen crosslinks in metabolic bone disease. Acta Orthop Scand 66: 171–175.
Eastell R 1994 Biochemical markers. Spine 8: 155–170.
Delmas PD 1995 Biochemical markers of bone turnover. Acta Orthop Scand 66: 176–182.
Delmas PD, Demiaux B, Malaval L, Chapuy MC, Edouard C, Meunier PJ 1986 Serum bone gamma carboxyglutamic acid containing protein in primary hyperparathyroidism and in malignant hypercalcemia. Comparison with bone histomorphometry. J Clin Invest 77: 985–991.
Eastell R, Colwell A, Hampton L, Reeve J 1997 Biochemical markers of bone resorption compared with estimates of bone resorption from radiotracer kinetic studies in osteoporosis. J Bone Miner Res 12: 59–65.
American Society for Clinical Nutrition/American Society for Parenteral and Enteral Nutrition Working Group on Standards for Aluminum Content of Parenteral Nutrition Solutions 1991 Parenteral drug products containing aluminum as an ingredient or a contaminant: response to Food and Drug Administration notice of intent and request for information. Am J Clin Nutr 53: 399–402.
ASPEN Board of Directors 1993 Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. J Parenter Enteral Nutr 17( suppl): 33: 38SA–38SA.
Colwell A, Russell RGG, Eastell R 1993 Factors affecting the assay of urinary 3-hydroxy pyridinium cross-links of collagen as a marker of bone-resorption. Eur J Clin Invest 23: 341–349.
Robins SP, Woitge H, Hesley R, Ju J, Seyedin S, Seibel MJ 1994 Direct, enzyme-linked immunoassay for urinary deoxypyridinoline as a specific marker for measuring bone resorption. J Bone Miner Res 9: 1643–1649.
LeGendre GR, Alfrey AC 1976 Measuring picogram amounts of aluminum in biological tissue by flameless atomic absorption analysis of a chelate. Clin Chem 22: 53–56.
Tsukahara H, Miura M, Hori C, Hiraoka M, Nosaka K, Hata K, Konishi Y, Sudo M 1996 Urinary excretion of pyridinium cross-links of collagen in infancy. Metabolism 45: 510–514.
Colwell A, Eastell R 1996 The renal clearance of free and conjugated pyridinium cross-links of collagen. J Bone Miner Res 11: 1976–1980.
Mora S, Prinster C, Bellini A, Weber G, Proverbio MC, Puzzovio M, Bianchi C, Chiumello G 1997 Bone turnover in neonates: changes of urinary excretion rate of collagen type I cross-linked peptides during the first days of life and influence of gestational age. Bone 20: 563–566.
Mayne PD 1994 Clinical chemistry of the newborn. In: Mayne PD (ed) Clinical Chemistry in Diagnosis and Treatment. Edward Arnold, London, 337–348.
Blumsohn A, Naylor KE, Assiri AMA, Eastell R 1995 Different responses of biochemical markers of bone resorption to bisphosphonate therapy in Paget disease. Clin Chem 41: 1592–1598.
Apone S, Lee MY, Eyre DR 1997 Osteoclasts generate cross-linked collagen N-telopeptides (NTx) but not free pyridinolines when cultured on human bone. Bone 21: 129–136.
Lapillonne A, Travers R, Dimaio M, Salle BL, Glorieux FH 1996 Bone remodeling assessed by urinary excretion of cross-linked N-telopeptides of type I collagen (NTx) in infants from birth to age one year. J Bone Miner Res 11:( suppl 1): S194(abstr).
Ott SM, Maloney NA, Klein GL, Alfrey AC, Ament ME, Coburn JW, Sherrard DJ 1983 Aluminum is associated with low bone formation in patients receiving chronic parenteral nutrition. Ann Intern Med 98: 910–914.
Koo WWK, Kaplan LA, Bendon R, Succop P, Tsang RC, Horn J, Steichen JJ 1986 Response to aluminum in parenteral nutrition during infancy. J Pediatr 109: 877–883.
Klein GL, Snodgrass WR, Griffin MP, Miller NL, Alfrey AC 1989 Hypocalcemia complicating deferoxamine therapy in an infant with parenteral nutrition-associated aluminum overload: evidence for a role of aluminium in the bone disease of infants. J Pediatr Gastroenterol Nutr 9: 400–403.
Acknowledgements
The authors are grateful to Dr. A. Blumsohn for his assistance in the measurement of serum osteocalcin, Dr. A. Colwell for his assistance in the measurement of the urinary cross-links by HPLC, and Nancy Miller for the measurements measurements of aluminum in urine and TPN solutions.
Author information
Authors and Affiliations
Additional information
*Presented in part at the Fifth Joint Meeting of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the North American Society for Pediatric Gastroenterology and Nutrition, Toulouse, France, May 27-30, 1998.
Rights and permissions
About this article
Cite this article
Naylor, K., Eastell, R., Shattuck, K. et al. Bone Turnover in Preterm Infants. Pediatr Res 45, 363–366 (1999). https://doi.org/10.1203/00006450-199903000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199903000-00012