Abstract
Retinopathy of prematurity (ROP) is characterized by inhibition of the growth of the retinal vessels and subsequent neovascularization. Pharmacologic doses of glucocorticoids are known to decrease growth and to suppress inflammation. The aim of the present study was to investigate whether hyperoxia and/or glucocorticoid affect the growth of the retinal vessels and the expression of the anti-inflammatory cytokine IL-1 receptor antagonist (IL-1ra). The following treatments were given to new-born rabbits during the rapid growth of retinal vessels: 1) placebo and room air (n = 14); 2) dexamethasone (Dx) at 1 mg/kg/d during d 3 to 8 and room air (n = 14); 3) placebo and 100% oxygen (d 3 to 7) (n = 14); 4) Dx and O2 (n = 16). On d 12, the eyes were studied for retinal vessel length and vascular surface area from India ink-perfused vessels. When indicated, retinas were harvested on d 7 and studied for the expression of IL-1ra mRNA using Northern blot analysis. Hyperoxia decreased the length and area of the retinal vessel complexes (p < 0.01) and induced neovascularization in three of eight animals (38%). Dx decreased the length and area (p < 0.01) and tended to increase the tortuosity of the retinal vessels. Dx did not potentiate the hyperoxia-induced suppression of retinal vessel growth and prevented the hyperoxia-induced neovascularization (p = 0.04). Hyperoxia inhibited the expression of IL-1ra mRNA, whereas Dx ameliorated the hyperoxia-induced suppression of IL-1ra. According to present results, glucocorticoid decreases the retinal vessel growth and may decrease the hyperoxia-induced neovascularization. We propose that immature and damaged retinal vessels are affected by pharmacologic dosage of glucocorticoid.
Similar content being viewed by others
Main
Advance in neonatal care is associated with increased survival rate of very low birth weight infants at risk of developing retinopathy of prematurity (ROP). According to one estimate, 420 infants per year experience vision loss as a result of ROP in the United States(1). More recently, Gibson et al.(2) estimated that 3400 infants suffer visual impairment from ROP each year while 650 will be blinded.
The inner retinal vasculature that nourishes the inner portion of the retina starts developing at 16 wk of human pregnancy and reaches the ora serrata at term(3). According to current hypothesis, two distinct pathologic features are characteristics of ROP. The inhibition in retinal vessel growth is an initial event, followed by neovascularization that is considered to be a repair response following the injury(4). Angiogenetic factors that contribute toward neovascularization, fibrosis and myogenesis, are formed around the boundary between vascular and avascular retina. This process may lead to retinal detachment and loss of vision.
It has been proposed that neonatal events, mainly the relatively hyperoxic environment, as well as hypoxia-reperfusion episodes induce vaso-obliteration and suppress growth of the retinal vessels(4–7). Hyperoxia, ischemia, and reperfusion in extraretinal organs is associated with inflammatory reaction and generation of inflammatory mediators including proinflammatory cytokines(8,9). Cytokines, produced by inflammatory cells, may exert toxic effects via production of oxygen radicals, lytic enzymes, and other mediators. Among them IL-1, a major inflammatory cytokine, stimulates the production of several other cytokines and up-regulates vasoactive mediators such as platelet-activating factor, prostaglandins, leukotrienes, nitric oxide, and vascular endothelial growth factor (VEGF)(8,10). VEGF is a mediator of inflammatory angiogenesis that may induce neovascularization of retinal vessel(11). IL-1 receptor antagonist (IL-1ra) is structurally related to IL-1 but functions as an inhibitor by competing with IL-1 for occupancy of the type 1 IL-1 receptor. IL-1ra does not induce signal transduction and therefore blocks various IL-1-induced responses(12,13).
Pharmacologic doses of glucocorticoid are often given to small preterm infants at risk of developing chronic lung disease. Glucocorticoid is known to suppress the growth of several organs, including brain(14). As part of the inhibitory role of glucocorticoid in inflammatory and immune processes, it may suppress the expression of IL-1 and of other cytokines(15,16).
Rodents are frequently used in experimental studies of retinal diseases. Rabbits have merangiotic (i.e. partly vascularized) retina. Vascularization starts at term and proceeds horizontally during the first two weeks of life(17). The newborn rabbit may thus serve as a model in studies on retinal vessel growth. The present aims were to study whether glucocorticoid inhibits retinal vessel growth or affects hyperoxia-induced retinal vascular changes in newborn rabbits.
METHODS
Protocol. Term New Zealand rabbit pups that had been nursed by the doe since birth were randomized at the age of 3 d to be placed in either 100% oxygen or room air. The two groups were further randomized to dexamethasone (Dx) or placebo (Pl) groups. Altogether, 65 animals were studied, 4 of them on d 3 of life. The rest were randomized into the following four treatment groups at the age of 3 d: 1) Pl, room air; 2) Dx, room air; 3) Pl, O2; 4) Dx, O2. Dexamethasone sodium phosphate (Merck Sharp Dohme, Rahway, NJ) 1 mg/kg/d or Pl was injected once a day intramuscularly during 5 d beginning d 3. Oxygen exposure lasted for 4 d. Thereafter, the pups were kept in room air for another 5 d and analyzed for retinal vessel growth, unless otherwise indicated. At 7 d of life, several hours after the allocated oxygen exposure was discontinued, 13 animals were euthanized, and eyes were collected for analysis of IL-1ra mRNA.
The animals were fed twice a day with Esbilac formula (120-160 mL/kg/d) using an orogastric feeding tube. The formula was fortified with lactalbumin (5 g/100 mL). During the feeding, all animals were kept in room air for about 1 min. The protocol was proven by the Institutional Review Board.
Study of retinal vessel growth. Four animals were studied at the age of 3 d. Altogether, 34 animals were euthanized at the age of 12 d. These animals underwent India ink perfusion for staining of retinal vessels. After euthanasia, thoracic cavity was opened, and a small catheter was placed through the left ventricular incision extending into the carotid artery. The vessels were perfused using Harvard syringe perfusion pump. Perfusion was done using 0.2% sodium nitroprusside in 0.9% saline followed by 50% India ink. The eyes were enucleated and placed in buffered 4% formalin fixative. Retinas of the fixed eyes were isolated and mounted in the glycerol gel on the slide. The retinas stained to the periphery with India ink were photographed from each animal. One retina from each animal was analyzed for vessel growth. The eye with the most complete staining was studied. Biometric data were collected either directly form the prints or by using image analysis program (Optimas) from the images transferred on compact disc.
All evaluations of the internal retinal vessel complexes were performed blindly without knowledge of the treatment allocation. The axial length of the anterior and posterior retinal vessel complexes was measured by one investigator (S.S.). The total length of the two vessel complexes was recorded. The area occupied by the vascular bed of both retinal vessel complexes was measured by one investigator (P.A.L.). Validity of measurements was assessed. Two randomly selected vessel complexes in each group were reanalyzed blindly by one investigator (M.H.). The variation coefficients of the paired measurements were calculated. The means ± SEM of the variation coefficients were 2.7 ± 0.7% (length) and 6.5 ± 1.6% (area), respectively.
Two investigators who were blinded to the study groups (P.A.L., M.H.) independently evaluated the vascular complexes for the following abnormalities. Neovascularization was defined as overgrowth beyond the borders of the vascular complex. Suspected neovascularization was defined as irregular vascular growth (no predictable direction, acute angulation, and variable caliber of vessels) within the vascular complex. There was a complete agreement between the two assessments. On the basis of inspection by the two investigators, the degree of vascular tortuosity (corkscrew appearance) was classified as "absent," "mild," or "increased." There was a complete agreement between the two assessments, except in one case ("mild" versus "increased" tortuosity after Dx, room air).
Isolation of retinas, RNA extraction, and Northern blot analysis of IL-1ra. The retinas were isolated in frozen state. RNA was isolated from retinas using guanidium isothiocyanate method(18). The RNA was quantitated at 260 nm, size separated in 1.2% agarose, 5% formaldehyde gel, transferred to a nylon hybridization membrane (Genescreen, NEN, Boston), and cross-linked onto the membrane by ultraviolet light. The membranes were hybridized using a 0.4-kb rabbit IL-1ra cDNA probe (a kind gift form Dr. S. Eisenberg, Synergen, Boulder, CO) that was random prime labeled with 32P. Prehybridization and hybridization were performed in a hybridization and hybridization were performed in a hybridization incubator. After posthybridization washes, the radioactivity indicating hybridization with the probe was visualized with x-ray film. To compensate for gel-loading artifacts, the membranes were additionally probed with 32P-radiolabelled rabbit mitochondrial CO II cDNA. The ratio of the densities, corresponding to IL-1ra and CO II mRNAs, was quantitated using video densitometry. Densitometric measurements were normalized to the corresponding measurements done on the control specimens (i.e. animals treated with placebo).
Statistics. The significance of the differences between the groups was analyzed using ANOVA. Multiple comparisons between the groups were performed using the least significant difference procedure of Fisher. The chi2 test was used for comparing the percentage vascular changes between the groups. A p less than 0.05 was considered to be significant. The results were expressed as means ± SEM.
RESULTS
Eleven animals died during the experiment (Pl: room air, n = 2; Dx: room air, n = 2; Pl: O2, n = 3; Dx: O2, n = 4; NS). The room air-treated animals gained more weight than the O2-treated ones. Dx treatment tended to decrease the weight gain, too. However, given the large variation and rather small number of animals studied, this was not statistically significant (Table 1).
On d 3, the total length of the anterior and posterior internal retinal vessels was 5.8 ± 1.7 mm (n = 4), and there was no (n = 3) or mild (n = 1) degree of vascular tortuosity. By 12 d of age, the length of the retinal vessels almost tripled (16.2 ± 0.5 mm, n = 8), and all vessel complexes became mildly tortuous. Both hyperoxia and Dx treatment inhibited the vessel growth (Table 2). The effects of hyperoxia and Dx were not additive. Both hyperoxia and Dx treatment decreased the vascular bed areas of retinal vessels. Again, these inhibitory effects were not additive.
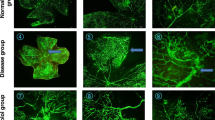
The groups receiving Dx revealed a trend toward increased vascular tortuosity [increased tortuosity in 8(7) of 18 versus 3 of 16; p = 0.15, χ2 test]. The corresponding figures for room air and hyperoxia-treated animals were 5 of 17 and 6 of 17, respectively. Neovascularization beyond the borders of the vascular complex was evident in three placebo-treated animals breathing oxygen (3/8); in three additional cases, the periphery of the vascular bed had irregular vasculature, suggesting neovascularization. However, none of the Dx-treated hyperoxic animals had evidence of neovascularization (0/9 versus 3/8; p = 0.04, χ2 test). Figure 1 shows representative retinal vessels in the five groups studied.
Retinal vascular complexes. The vascular complex from a 3-d-old animal its scale are shown in the center. The four other vascular complexes and their scale (right lower corner) are from 12-d-old animals treated with dexamethasone (DEX: 1 mg/d during d 3 to 8)/placebo and 100% oxygen during d 3 to 7/room air (RA). Both dexamethasone and O2 decreased the growth of the vascular complex (see also Table 2). Retina from placebo and O2-treated animal reveals faint neovascularization beyond the border of the vascular bed (arrow) and disordered vessels in peripheries of the vascular complexes. Retinas from the dexamethasone-treated animals show a trend toward increased vascular tortuosity, but there is no neovascularization.
Exposure to hyperoxia decreased the expression of IL-Ira mRNA by a mean of 72%. Dx alone had no detectable effect on IL-Ira expression. However, Dx decreased the suppression of IL-Ira during hyperoxia (Table 3).
DISCUSSION
According to experimental studies, both hyperoxemia and hypoxia affect the development of retinal vessels(5,7,8). The decrease in vascular growth induced by oxygen is proposed to be the result of suppression of VEGF causing apoptosis of endothelial cells in immature retina (11). Ashton et al.(19,20) demonstrated in a premature feline model that hyperoxia leads not just to constriction of the retinal vessels but to obliteration making the retina avascular. Consequently, the inner layers of the retina are exposed to ischemia that may be complicated by intermittent hypoxemia. This results in uncontrollable upregulation of VEGF, inducing proliferative vascularization and increased permeability that are characteristic features of ROP(11,21,22). Exposure to hyperoxia followed by breathing of room air, as shown in present and other(5,7) studies, decreased the normal growth of the retinal vessels and increased the incidence of neovascularization.
In ROP, similar to inflammatory conditions(10,23,24), the increase in VEGF could be the result of an increase in IL-1 activity. This may be because of either an increase in proinflammatory IL-1 or a decrease in anti-inflammatory IL-1ra activity. The present study demonstrated suppression of the anti-inflammatory cytokine IL-1ra following hyperoxia. Although this finding supports the possibility that exposure to hyperoxia is associated with increased retinal IL-1 activity, its relationship with retinal vessel growth remains uncertain.
Glucocorticoid is often given to small preterm infants for treatment of respiratory failure and for prevention of chronic lung disease. Despite many randomized trials, the optimal dosage and indications of glucocorticoid therapy remain open. Many side effects of glucocorticoid given to the premature (inhibition of growth, infections, cardiomyopathy, neurodevelopmental problems, and others) have been reported. Concerns have been raised about the harmful effects of glucocorticoid treatment on immature retina of small premature infants(25,26).
In this study, we have shown for the first time that a pharmacologic dose of Dx (1 mg/kg/d for 5 d) inhibited the growth of retinal vessels. The vessel growth inhibition by Dx was evident when the animals were kept in room air. However, Dx did not potentiate the hyperoxia-induced inhibition of retinal vessel growth. The finding that with Dx there was no evidence of neovascularization among animals exposed to oxygen of neovascularization among animals exposed to oxygen raises the possibility that glucocorticoid also suppresses abnormal retinal vessel growth caused by hyperoxia.
According to current hypothesis, ROP is characterized as a primary growth failure followed by abnormal proliferation of retinal vessels. It has been proposed that the final pathway of neovascular growth is regulated by VEGF(11). Cytokines act synergistically or in opposition with the growth factors or orchestrate the development, progression, and resolution of the wound-healing response. Proinflammatory cytokines have been detected in the vitreous and aqueous fluid of patients with proliferative diabetic retinopathy and in other inflammatory conditions(27,28). Oxygen as shown in our experiment decreased the expression of IL-mRNA. Glucocorticoid has been shown to decrease IL-1ra synthesis following induction by endotoxin in isolated monocytes(29). In our experiment, glucocorticoid alone had no effect on IL-1ra expression in retina in vivo. However, Dx treatment decreased the suppression of IL-1ra by hyperoxia. This finding supports the concept that glucocorticoid serves as an anti-inflammatory agent following hyperoxic damage of retina, suppressing the activity of IL-1 that is known to up-regulate VEGF(10).
The pattern of growth inhibition of retinal vascular complex exposed to Dx (particularly lack of neovascularization) and the lack of detectable suppression of the anti-inflammatory cytokine IL-1ra were the features different from abnormal retinal vessel growth owing to hyperoxia. The present result is likely to be dependent on the dose, duration, and onset of glucocorticoid as well as on species. The present result raises concerns about the growth suppression of immature retinal vessels during during glucocorticoid treatment as a factor contributing to ROP(25,26). It also points out the potential of glucocorticoid in decreasing the neovascularization. The effect of glucocorticoid on retinal vessel growth and incidence/severity of ROP remain to be further evaluated in clinical trials.
Abbreviations
- Dx :
-
dexamethasone
- ROP :
-
retinopathy of prematurity
- IL-1ra :
-
IL-1 receptor antagonist
- Pl :
-
placebo
- VEGF :
-
vascular endothelial growth factor
References
Phelps DL 1981 Retinopathy of prematurity: an estimate of vision loss in the U.S.-1979. Pediatrics 67: 924–925.
Gibson D, Sheps S, Uh SH, Schetchter M, McCormick A 1990 ROP-induced blindness: birthweight-specific survival and the new epidemic. Pediatrics 86: 405–412.
Ashton N 1970 Retinal angiogenesis in the human embryo. Br Med Bull 26: 103–106.
Chang-Ling T, Stone J 1993 Retinopathy of prematurity: origins in the architecture of the retina. Prog Retin Eye Res 12: 155–178.
Michaelson C, Herz N, Lewkowitz E, Kertesz D 1954 Effect of increased oxygen on the development of the retinal vessels. Br J Ophthalmol 38: 577–587.
Penn JS, Tolman BL, Lowery LA 1993 Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci 34: 576–585.
Ashton N 1996 Oxygen and the growth and development of retinal vessels. Am J Ophthalmol 96: 413–435.
Dinarello CA 1991 Interleukin-1 and IL-1 antagonism. Blood 77: 1627–1652.
Saugstad OD 1996 Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics 98: 103–107.
Ben-Av P, Crofford LJ, Wilder RL, Hla T 1995 Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett 372: 83–87.
Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E 1995 Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature Med 1: 1024–1028.
Dinarello CA, Thompson RC 1991 Blocking IL-1: IL-1ra in vivo and in vitro. Immunol Today 12: 404–415.
Arend WP 1991 Interleukin-1 receptor antagonist: a new member of the interleukin-1 family. J Clin Invest 88: 1445–1451.
Meyer JS 1985 Biochemical effects of corticosteroids on neural tissues. Physiol Rev 65: 946–1020.
Besedowsky H, Rey A, Sorkin E, Dinarello CA 1986 Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233: 652–655.
Monick MM, Aksamit TR, Geist LJ, Hunninghake GW 1994 Dexamethasone inhibits IL-1 and TNF activity in human lung fibroblasts without affecting IL-1 or TNF receptors. Am J Physiol 267:L33–L38.
Ashton N, Tripathi B, Knight G 1972 Effect of oxygen on the developing retinal vessels of the rabbit: I. anatomy and development of retinal vessels of the rabbit. Exp Eye Res 14: 214–220.
Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159.
Ashton NA, Cook C 1955 Studies on the developing retinal vessels: I. influence of retinal detachment. Br J Ophthalmol 39: 449–454.
Ashton NA, Cook C 1955 Studies on the developing retinal vessels: II. influence of retinal detachment on oxygen vaso-obliteration. Br J Ophthalmol 39: 457–462.
Levy AP, Levy NS, Goldberg MA 1996 Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem 271: 2746–2753.
Aiello L, Northrup J, Keyt B, Takagi H, Iwamoto M 1995 Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 113: 1538–1544.
Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E 1995 Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest 72: 638–645.
Jackson JR, Minton JA, Ho ML, Wei N, Winkler JD 1997 Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and interleukin 1beta. J Rheumatol 24: 1253–1259.
Wright K, Wright SP 1994 Lack of association of glucocorticoid therapy and retinopathy of prematurity. Arch Pediatr Adol Med 148: 848–852.
Ramanathan R, Siassi B, De Lemos RA 1995 Severe retinopathy of prematurity in extremely low birth weight infants after short-term dexamethasone therapy. J Perinatol 15: 178–182.
Franks W, Limb G, Stanford M, Ogilvie J, Wolstencroft R, Chignell A, Dumonde D 1992 Cytokines in human intraocular inflammation. Curr Eye Res 11:( suppl): 187–191.
Kauffman D, Meurs Jan, Mertens D, Peperkamp E, Master C, Gerrilse M 1994 Cytokines in vitreous humor: interleukin-6 is elevated in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 35: 900–906.
Arzt E, Sauer J, Pollmacher T, Labeur M, Holsboer F, Reul J, Stalla G 1994 Glucocorticoids suppress interleukin-1 receptor antagonist synthesis following induction by endotoxin. Endocrinology 134: 672–677.
Author information
Authors and Affiliations
Additional information
Supported by the Finnish Academy and Orange County Infant Care Specialists (OCICS) Research Foundation.
Rights and permissions
About this article
Cite this article
Lawas-Alejo, P., Slivka, S., Hernandez, H. et al. Hyperoxia and Glucocorticoid Modify Retinal Vessel Growth and Interleukin-1 Receptor Antagonist in Newborn Rabbits. Pediatr Res 45, 313–317 (1999). https://doi.org/10.1203/00006450-199903000-00004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199903000-00004
This article is cited by
-
Impact of postnatal steroids on peripheral avascular retina and severity of retinopathy of prematurity
Pediatric Research (2023)