Abstract
We asked whether repeated hypoxic exposures during the early neonatal periods could affect the ventilatory control, such as the lung volume-dependent ventilatory inhibition (HBR), pulmonary ventilation (V˙E), and CO2 production (V˙CO2). Within each litter of rats, one group of pups (experimental group H) was exposed to 6% O2 (30-min duration twice a day from postnatal d 1 to 4). The other group (control group C) was exposed to air. At 5 d after birth, the HBR was triggered by lung inflation via negative body surface pressure (10 cm H2O). Measurements of V˙E and V˙CO2 were done by plethysmography and the inflow-outflow CO2 difference, respectively. At 2 wk of age, V˙E and V˙CO2 measurements were repeated by the barometric technique and the inflow-outflow CO2 difference, respectively. Each conscious pup was breathing normoxia (21% O2) and then hypoxia (10% O2). Results were as follows: 1) during normoxia, HBR was stronger and both V˙E and V˙CO2 were higher in H pups than in C pups; 2) during hypoxia, the HBR of C was as in normoxia, whereas that of H was increased above the normoxic value; 3) during hypoxia, C maintained V˙E, whereas H decreased it; 4) in hypoxia, V˙CO2 was reduced significantly in both groups; 5) at 2 wk of age, V˙E and V˙CO2 did not differ between H and C during normoxia or in response to 10% hypoxia. We conclude that in rat pups, repeated hypoxic episodes can modify the HBR and, at least temporarily, reduce the V˙E response to hypoxia with a decrease in V˙CO2. The findings are in agreement with the view that repeated hypoxic exposures in the neonatal period could interfere with the development of respiratory control and could possibly be involved in the mechanisms of neonatal apnea or sudden infant death syndrome.
Similar content being viewed by others
Main
Sustained or repeated episodes of hypoxia can be observed frequently during the newborn period. For instance, sustained hypoxia due to chronic lung disease, e.g. bronchopulmonary dysplasia, or repeated hypoxia from recurrent apnea often occurs during this period. As for the effect of sustained hypoxia on postnatal ventilation, infants born at high altitude and animals reared in a sustained low O2 environment are known to have a blunted ventilatory response to hypoxia(1–3). Although the blunting seems to be in part at the level of the carotid body, the precise mechanism of the arterial chemoreceptor resetting process is still unknown. On the other hand, the effects of repeated episodes of hypoxia during the early neonatal period have never been studied.
In the present study, we aimed to determine whether the respiratory control of conscious newborn rats would be affected by preceding experience with repeated hypoxia during early postnatal ages. We measured the HBR, which is mediated by the vagi via stimulation of the stretch receptors in the pulmonary airways and is of major importance in the control of the depth and duration of inspiration in human newborns and animals(4). We regarded the intensity of HBR as an index of vagal chemosensory interaction between the vagal inhibition and chemostimulants (low PaO2 and high PaCO2)(5). In addition, we measured V˙CO2 and ventilation because the reduction in V˙CO2 could decrease the ventilatory drive(6). We hypothesized that repeated hypoxia in newborns may decrease metabolic rate and that the vagal ventilatory inhibition could be more effective. Hence, in the newborn, the effect of repeated hypoxic exposure on the ventilatory response to lung inflation could be greater than in controls because of decreased chemosensitivity or lower metabolic drive.
METHODS
Experiments were performed on 90 Sprague-Dawley newborn and young rats (11 litters) in accordance with the guidelines for animal experimentation of the Shinshu University School of Medicine. All animals were mother reared and maintained on 12:12-h dark-light cycle (lights on from 0900 to 2100) at Tamb of 24 ± 1 °C and 50 ± 10% air relative humidity.
Within each litter, pups were randomly assigned to one of two groups. One group was repeatedly exposed to hypoxia (H); the other group was never exposed to hypoxia (C). Hypoxic exposures consisted of breathing 6% O2 for 30 min twice a day, in the morning and evening from d 1 to 4 after birth. During the exposures, pups were placed in a 965-mL Plexiglas chamber at Tamb = 28-30°C. A flow (2000 mL/min) of air for C pups or hypoxia for H pups, obtained by blending air and N2, was delivered through the chamber as the inflow gas concentration was monitored by an O2 and CO2 gas analyzer (NEC San-Ei Instruments, 1H26, Tokyo, Japan). The C pups were treated identically to the H pups, except that they were breathing air instead of 6% hypoxic gas. After the exposure, pups were returned to their dams and housed in cages in a temperature-controlled room.
The main protocol consisted of measurements of the HBR, V˙CO2, and ventilation during acute hypoxia (10% O2 in N2) or air (21% O2) in H and C at d 5 after birth. A first set of experiments was done to evaluate the effects on the HBR. V˙CO2 was measured in a second group and then the ventilatory response was measured in a third group of pups. In addition, in 9 of C rats [body weight (BW) = 10.5 ± 0.5 g] and 10 of H pups (BW = 9.9 ± 0.3 g), 3% CO2 was added to the hypoxic mixture at the end of the hypoxia during the measurement of the breathing pattern to test their ability to further increase V˙E. Finally, in a fourth set, the measurements of breathing pattern and V˙CO2 were performed at 2 wk after birth. In all cases, recordings began after 10 to 15 min of the normoxic or hypoxic exposures after the animal was resting quietly, possibly asleep.
The setup for 5-d-old pups consisted of a double-chamber body plethysmograph similar to that previously described(7). The animal was resting in the back chamber while the head was emerging through four layers of paraffin sealing film (Parafilm) into the front chamber (Fig. 1A). The front chamber, for measurements of the breathing pattern and V˙CO2, also permitted the delivery of air, hypoxic, or hypoxic-hypercapnic gases(8). A homemade pneumotachograph connected to a differential pressure transducer (NIHON KOHDEN, TP-602T, Tokyo, Japan) was attached to the front chamber for measurements of V˙ (NIHON KOHDEN, AR-601G) and VT by electronic integration (NIHON KOHDEN, AQ-601G). The back chamber was connected to a vacuum source for the application of negative body surface pressures. The pressure of this chamber was monitored by a pressure transducer (NIHON KOHDEN, TP-603T). Tamb in the back chamber was continuously monitored by tungsten-constantan thermocouple (Omega, 871A, Stamford, CT) and maintained at thermoneutrality (32-34°C) with a heating lamp. Colonic temperature of 5-d-old pups was not monitored to leave their vulnerable colonic mucosa intact.
(A) Schema of setup. Breathing pattern (airflow, V˙ and VT) was monitored via a handmade pneumotachograph connected to the front chamber. The O2 and CO2 concentrations of a steady flow of gas (air, hypoxia, or hypoxia-hypercapnia) through the front chamber were monitored. A syringe connected to the front chamber was for calibration of VT by injection and withdrawal of gas volumes in known amounts. DP, pressure transducer; insp, inspiratory; exp, expiratory. (B) Records of V˙ (inspiration upward), VT, and pressure (P) at body surface of newborn rat during resting breathing. As P was lowered, lung volume increased and inspiratory activity ceased (Hering-Breuer reflex) until the chemical drive triggered an inspiratory effort. TEc, control expiratory time; TEinfl, inflation expiratory time.
Measurements of the HBR. At the end of expiration, a negative body surface pressure of 10 cm H2O was applied; as the result, lung volume increased and breathing temporarily ceased (Fig. 1B). The pressure was released immediately after the first inspiratory effort during lung inflation. Vacuum pressure, V˙, and VT were recorded on paper. The HBR was quantified in a form of inhibitory ratio (IR)(9). From the V˙ record, control expiratory time was calculated and averaged for the three breaths before the inflation; the inflation expiratory time was the time interval between the end of the inspiration before the inflation and the onset of the first inspiratory effort during the inflation. Hence, IR corresponded to inflation expiratory time/control expiratory time. In each pup, data represent the average of three inflations.
Measurements of V˙CO2. A steady flow of gas (100 mL/min at 5 d or 600 mL/min at 2 wk) was continuously delivered through the chamber and controlled by a fine flowmeter. Outflowing CO2 concentration was monitored by an infrared CO2 analyzer after passing through a drying column (Drierite). Gas concentration was displayed on a computer monitor during on-line acquisition. V˙CO2 was computed as the inflow-outflow gas concentration difference averaged over 1-3 min and multiplied by the gas flow. Values of V˙CO2 are presented as standard temperature, pressure, dry and normalized by the BW of the animal.
Measurements of ventilatory responses. The ventilatory response for 5-d-old pups was measured by flow plethysmography(8,10). Young male rats, 14- to 15-d-old, were placed in a 230-mL Plexiglas chamber. A flow of the desired inspiratory gases was continuously delivered through the chamber, where Tamb was maintained at 25 ± 1 °C. Colonic temperature was monitored by a tungsten-constantan thermocouple. The flow was momentarily stopped by sealing the chamber, and the breathing pattern was measured by a barometric technique(11,12); the oscillations in chamber pressure were monitored by a pressure transducer (NIHON KOHDEN, TP-603T) and converted to volume by appropriate correction factors(11). Data of breathing patterns were based on measurements of 100 breaths for 5-d-old rats and 50 breaths for 2-wk-old rats; volume data, i.e. VT, VT/TI (the ventilatory equivalent of the neural inspiratory drive), and V˙E (as VT × respiratory frequency) were calculated at body temperature, pressure, water vapor saturated and normalized by the weight of the animal. The record was analyzed with the help of a graphics tablet connected to a personal computer.
Data analysis and statistics. All values are presented as mean ± SEM. In addition to raw data, percent changes of hypoxia from normoxia [(hypoxic values-normoxic values)/normoxic values × 100] were used to compare the degree of the response to 10% hypoxia between the two groups. Significance of the differences between the two groups in normoxia and percent changes were evaluated with a 2-tailed independent t test. Between normoxia and 10% hypoxia within each group, statistical comparisons were made by a 2-tailed paired t test. Further, for the response to 10% hypoxia, significant differences between average data of the sequential measurement performed on the same animals were assessed by repeated measures 1-way ANOVA with mixed design in which one variable is a treatment group with two levels (C versus H) and the other variable is an experimental condition with two levels (FIO2 = 0.21 versus 0.10). In all cases, the level of significance was considered for p < 0.05.
RESULTS
The breakdown of the animals studied for each measurement is presented in Table 1. No deaths occurred during the experimental periods in either C or H group.
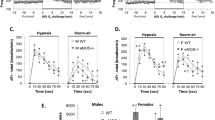
HBR. In normoxia, the IR of H pups was significantly greater than those of C pups (Fig. 2). During hypoxia, the IR of H further increased above the normoxic value, whereas that of C was unchanged. By repeated ANOVA, the two groups had significantly different IR both in air and hypoxia.
IR of 5-d-old rats repeatedly exposed to 6% inspired O2 (H, ·) and air (C, ○). In normoxia, IR was significantly stronger in H than in C. In hypoxia, IR of H was significantly increased above the normoxic value, whereas IR of C did not change. Overall, the two groups had significantly different IR. Values are means of 1 SEM. *p < 0.05 between C and H by unpaired t test; †p < 0.05 between normoxia and 10% hypoxia by paired t test; §between C and H by repeated ANOVA.
V˙CO2 at 5 d. During normoxia, V˙CO2 was significantly higher in the H group compared with the C group. With 10% hypoxia, V˙CO2 was significantly reduced in both groups (Table 2). Although there was no significant difference between the two groups by repeated ANOVA, the percent change from normoxia in H group (-31.7 ± 4.6%) was statistically greater than in C group (-17.3 ± 3.7%).
Breathing patterns at 5 d. In normoxia, VT/TI and V˙E of the H animals were significantly greater than those of the C animals (Fig. 3,A and B). On the other hand, neither VT nor timing parameters such as inspiratory time, total respiratory cycle, inspiratory time as a fraction of the total respiratory cycle, and respiratory frequency differed between the two groups during normoxia (Table 2). Further, although repeated ANOVA on V˙E or VT/TI did not show significant differences between the two groups, the percent changes in H group were significantly greater than in the C group (Fig. 3,A and B).
Ventilatory output (A, V˙E and B, VT/TI) of 5-d-old rats repeatedly exposed to 6% inspired O2 (H, ·) and air (C, ○). These two parameters had the same statistical results. In normoxia, they were significantly higher in H than in C. In hypoxia, the values of both parameters significantly decreased below normoxic values in the H but not in the C group. The percent changes in the H group were significantly higher than in the C group. Values are means of 1 SEM. Mean percent changes + SEM are in parentheses. *p < 0.05 between C and H by unpaired t test; †p < 0.05 between normoxia and 10% hypoxia during normoxia by paired t test; **p < 0.05 between C and H by unpaired t test.
During hypoxia-hypercapnia combined, V˙E (mL · kg-1 · min-1) significantly exceeded the hypoxic value, from 1079 ± 41 to 1625 ± 61 in the H pups and from 1084 ± 53 to 1478 ± 68 in the C pups.
Breathing patterns and V˙CO2 at 2 wk. All parameters of breathing and V˙CO2 at 2 wk did not differ significantly between the two groups during normoxia. VT/TI and V˙E increased significantly on exposure to 10% hypoxia in both groups with no significant difference (Table 3).
V˙E-V˙CO2 relationship. Figure 4 summarizes the changes in V˙CO2 and the corresponding changes in V˙E in the 5-d-old (A) and 2 wk-old (B) rats. At 5 d, during normoxia, V˙CO2 of the H group increased above the value of the C group with a parallel increase in V˙E, to the extent that the V˙E-V˙CO2 ratio was almost unchanged (Fig. 4A). By contrast, at 2 wk, there were no differences between the two groups during air breathing (Fig. 4B). The V˙E-V˙CO2 response to hypoxia had a different pattern between the two groups. At 2 wk of age, breathing 10% O2 decreased V˙CO2 and increased V˙E. In the 5-d-old rats, hypoxia decreased V˙CO2 in both groups with a decrease in V˙E in H and no change in C.
V˙E-V˙CO2 relationship of (A) 5-d-old and (B) 2-wk-old rats repeatedly exposed to 6% inspired O2 (H, ·). A, At 5 d, during normoxia, V˙CO2 of H was above the value of C, paralleled by an increase in V˙E to the extent that the V˙E-V˙CO2 ratio was almost unchanged. B, At 2 wk, there were no differences between the two groups during air breathing. During 10% hypoxia, there were different patterns on V˙E-V˙CO2 between the two ages. At 2 wk, hypoxia decreased V˙CO2 with an increase in V˙E. At 5 d of age, in hypoxia, V˙CO2 decreased with a decrease in V˙E (H group) but with no change in V˙E (C group).
DISCUSSION
To understand the pathophysiology of diseases responsible for respiratory dysregulation such as SIDS or apnea of prematurity, it is desirable to know to what extent neonatal hypoxia may affect the postnatal development of respiratory control. However, it is difficult to understand these effects because of differences in the magnitude, frequency, and duration of the hypoxic exposures. We assessed the intensity of the HBR in the form of IR after lung inflation(9); we found that daily repeated hypoxic exposures resulted in an increased IR compared with nonhypoxic-exposed pups. These results showed that repeated hypoxia during the early neonatal period affected the development of respiratory control in newborn rats. This intriguing phenomenon could not be fully explained by differences in metabolic drive and chemosensitivity.
Before examining the implication of the present results on ventilatory control, it is important to consider the potentially confounding factors introduced by 1) arousal, behavioral, and sleep conditions, 2) interpretation of neural output from ventilatory parameters, and 3) the role of respiratory muscle fatigue(13). First, it is important to know whether increased irritability and arousal occurred as the result of the repeated hypoxic exposure. For instance, if H group were more irritable than C group, V˙E and V˙CO2 might be higher and lung inflation reflex might be less potent in H than in C. However, IR was stronger in H than C during air breathing and IR of H in hypoxia was increased above the normoxic value. Therefore, it seems unlikely that repeated hypoxic exposure affected behavioral characteristics, including irritability. Previous literature(14) showed that although newborn rats spend approximately 70% of the time asleep, rapid eye movement and nonrapid eye movement sleep stages cannot be differentiated in terms of electrocortical activity until approximately 2 wk. Despite the fact that it is very difficult to determine the arousal and sleep levels in newborn rats, we have chosen to study our pups in the conscious state to avoid the depressant effects of anesthetics or sedatives on the ventilatory control system(15). The translation of neutral output into ventilatory events depends on inspiratory muscle strength, rib cage rigidity and compliance, airway resistance, and lung compliance. The status of these parameters as well as their integrative functioning at any given time affects the performance of the respiratory pump and is mediated by specific reflex arcs(16). Massaro et al.(17) have reported that even short-term hypoxic exposures immediately after birth may alter postnatal development of the lung structure of rats. Therefore, it could be of interest to study the difference of lung morphology and respiratory mechanics between the two groups to evaluate to what extent they may have contributed to the ventilatory differences. However, the HBR in this study should not have been affected by differences in the mechanical properties of the respiratory system. In fact, inflations were performed at constant pressures, not volumes, since the former, not the latter, is the appropriate stimulus for the airway stretch receptors(18). Finally, we observed that, during hypoxia-hypercapnia combined, VT, VT/TI, and V˙E increased above the hypoxic values in both the C and H group. This should indicate that there were no major limitations in the mechanical performance of the respiratory system, including the respiratory muscles.
In the 5-d-old rats, we found that IR of C in hypoxia was as in normoxia, confirming a previous report(7). On the other hand, in rats with daily repeated hypoxic exposures, HBR during 10% O2 was stronger than in normoxia. This may be because of the exceptionally strong HBR in H pups, because IR was stronger in H than in C even in normoxia. The HBR plays a fundamental role in regulation of the breathing pattern in animals and human newborns(19). Younes et al.(5) showed that the termination of the apnea after lung inflation is a function of the increasing chemoreceptor drive, i.e. increasing PaCO2 and decreasing PaO2. In other words, the stimulatory chemical drive eventually breaks the vagal inhibition because hypoxia and hypercapnia gradually develop with a time course that depends on metabolic rate. Hence, the HBR, quantified as IR, is expected to be modulated by chemosensitivity and the rate of V˙CO2. In fact, hypoxia decreased metabolic rate, which is a phenomenon known to occur especially in newborns(20). This should imply a reduction in ventilatory drive, possibly via the decrease in V˙CO2(6), and the vagal ventilatory inhibition during lung inflation would therefore be stronger than in absence of the hypometabolic response. Hence, more pronounced hypometabolism in H rats could have contributed to the intensity of IR to the chemical stimuli. However, we did not obtain clear evidence for interaction between V˙CO2 and IR in the present study.
One would expect that continuation of the repeated hypoxic insults in newborns could result in major changes in respiratory, cardiovascular, or hematological functions, oriented to adapt the newborn to the hypoxic condition and to protect postnatal development. Several investigators have studied the structural and functional respiratory changes during chronic hypoxia within the neonatal period. For instance, Okubo and Mortola(21) have reported O2 consumption and V˙E to be above control in the first week after chronic hypoxia in rats. They speculated that these phenomena may reflect the higher O2 demands of the rapidly growing animals during the "catch up growth" after the hypoxia. In our experiments, pups experienced repeated hypoxia-elevated V˙CO2 and V˙E with no effects on BW. We also found that the ventilatory response to 10% O2 was less in H than C. Okubo and Mortola(21) also showed in rats exposed to sustained hypoxia during the first week of life that V˙E remained elevated even 7 wk after birth, at a time when BW and O2 consumption had reached the control values, and the ventilatory response to acute hypoxia was still blunted in 7-wk-old rats. Differently, in the present study, the effect of repeated hypoxic exposure on the regulation of breathing was temporary, because no differences were observed at 2 wk after birth. It is likely that long-lasting effects on the control of breathing differ between sustained hypoxia and repeated hypoxia.
In conclusion, in the rats with daily hypoxic episodes during the early postnatal period, V˙E was higher than in control rats; the ventilatory response to acute hypoxia was blunted and the lung volume-dependent ventilatory inhibition was stronger. This may suggest that the combination of decreased sensitivity to the inputs from the chemoreceptor coupled to a reduced central nervous inhibition on inspiratory activity may contribute to ventilatory depression in newborn rats that experienced repeated hypoxic insults.
Abbreviations
- HBR:
-
Hering-Breuer inflation inhibitory reflex
- V˙CO2:
-
carbon dioxide production
- PaO2:
-
arterial oxygen pressure
- PaCO2:
-
arterial carbon dioxide pressure
- V˙E:
-
minute ventilation
- VT:
-
tidal volume
- V˙:
-
respiratory flow
- VT/TI:
-
mean inspiratory airflow
- Tamb:
-
ambient temperature
References
Sørensen SC, Severinghaus JW 1968 Irreversible respiratory insensitivity to acute hypoxia in man born at high altitude. J Appl Physiol 25: 217–220
Sladek M, Grogaard JB, Parker RA, Sundell HW 1993 Prolonged hypoxemia enhances and acute hypoxemia attenuates laryngeal reflex apnea in young lambs. Pediatr Res 34: 813–820
Sladek M, Parker RA, Grogaard JB, Sundell HW 1993 Long-lasting effect of prolonged hypoxemia after birth on the immediate ventilatory response to changes in arterial partial pressure of oxygen in young lambs. Pediatr Res 34: 821–828
Widdicombe JG 1964 Respiratory reflexes. In: Handbook of Physiology. Respiration. American Physiological Society, Washington, DC, pp 585–630
Younes M, Vaillancourt P, Milic-Emili J 1974 Interaction between chemical factors and duration of apnea following lung inflation. J Appl Physiol 36: 190–201
Mortola JP, Matsuoka T 1993 Interaction between CO2 production and ventilation in the hypoxic kitten. J Appl Physiol 74: 905–910
Matsuoka T, Mortola JP 1995 Effects of hypoxia and hypercapnia on the Hering Breuer reflex of the conscious newborn rat. J Appl Physiol 78: 5–11
Saetta M, Mortola JP 1985 Breathing pattern and CO2 response in newborn rats before and during anesthesia. J Appl Physiol 58: 1988–1996
Widdicombe JG 1961 Respiratory reflexes in man and other animals. Clin Sci 21: 163–170
Saetta M, Mortola JP 1987 Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol 62: 506–512
Bartlett D Jr, Tenney SM 1970 Control of breathing in experimental anemia. Respir Physiol 10: 384–395
Frappell P, Lanthier C, Baudinette RV, Mortola JP 1992 Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol 262:R1040–R1046
Bryan AC, Bowes G, Maloney JE 1986 Control of breathing in the fetus and the newborn. In: Fishman AP, Cherniack NS, Widdicombe JG, Geiger SR (eds) The Respiratory System (Section 3). American Physiological Society, Bethesda, MD, pp 621–647
Jouvet-Mounier D, Astic CL, Lacote D 1969 Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Devl Psychobiol 2: 216–239
Bouverot P, Crance JP, Dejours P 1970 Factors influencing the intensity of the Breuer-Hering inspiration-inhibiting reflex. Respir Physiol 8: 376–384
Davis GM, Bureau MA 1987 Pulmonary and chest wall mechanisms in the control of respiration in the newborn. Clin Perinatal 14: 551–579
Massaro GD, Oliver J, Massaro D 1989 Short-term perinatal 10% O2 alters postnatal development of lung alveoli. Am J Physiol 257:L221–L225
Gaultier C, Mortola JP 1981 Hering-Breuer inflation in young and adult mammals. Can J Physiol Pharmacol 59: 1017–1021
Sankaran K, Leahy FN, Cates D, MacCallum M, Rigatto H 1981 Effect of lung inflation on ventilation and various phases of the respiratory cycle in preterm infants. Biol Neonate 40: 160–166
Mortola JP, Gautier H 1994 Interaction between metabolism and ventilation: effects of respiratory gases and temperature. In: Dempsy JA, Pack AI (eds) Regulation of Breathing. Dekker, New York, pp 1011–1064
Okubo S, Mortola JP 1990 Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol 259:R836–R841
Acknowledgements
The authors thank Drs. Jacopo P. Mortola (Professor, Department of Physiology, McGill University, Montreal, Canada) and Shuichi Okubo (Director, Department of Chest Diseases, Yamanashi Prefectural Central Hospital) for their helpful discussion.
Author information
Authors and Affiliations
Additional information
Supported by a Grant-in-Aid (07671273) from the Ministry of Education and by a Grant for Pediatric Research (8C-02) from the Ministry of Health and Welfare.urrent address [T.M.]: Department of Nursing, Shinshu University School of Allied Medical Sciences, 3-1-1 Asahi, Matsumoto, Nagano 390-8621, Japan.
Rights and permissions
About this article
Cite this article
Matsuoka, T., Yoda, T., Ushikubo, S. et al. Repeated Acute Hypoxia Temporarily Attenuates the Ventilatory Respiratory Response to Hypoxia in Conscious Newborn Rats. Pediatr Res 46, 120–125 (1999). https://doi.org/10.1203/00006450-199907000-00020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199907000-00020