Abstract
Previous data suggested that small for gestational age newborns have increased levels of IGF binding protein 1 (IGFBP1) in amniotic fluid (AF) at 15-16 wk of pregnancy. In this study, we developed an RIA for IGFBP1 and measured IGFBP1 concentrations in 209 AF samples with normal fetal karyotype between 14 and 20 wk; we measured IGF-I, IGF-II, and C-peptide in the same samples. Concentrations of these growth-modulating factors were all positively correlated with gestational age at sampling (p < 0.0001). After correcting for gestational age, AF IGFBP1 remained strongly correlated with IGF-I and IGF-II (both p < 0.0001); their concentrations were many times higher in AF than in cord serum during the third trimester. None of the growth-modulating factors in AF correlated with birth weight, after correction for gestational age; birth weight percentile distribution was comparable in two groups of newborns who had AF values of IGF-I, IGF-II, IGFBP1, or C-peptide that were either less than or equal to the 50th percentile or more than the 50th percentile at sampling. However, placenta weight and the placenta weight to birth weight percentage were negatively correlated with AF IGF-I, IGF-II, and IGFBP1; placenta weight to birth weight percentage was lower in pregnancies with IGFBP1 values more than the 50th percentile compared with those less than or equal to the 50th percentile at sampling. In conclusion, AF concentrations of IGFBP1 increase gradually between 14 and 20 wk gestational age and correlate with IGF-I and IGF-II levels; high IGFBP1 levels do not predict small for gestational age newborns, but are associated with lower placenta weight.
Similar content being viewed by others
Main
Insulin and IGF (IGF-I and IGF-II) are important regulators of fetal growth. Insulin is synthesized in the fetal pancreas from 11 to 12 wk onward(1), and presumably acts in an endocrine mode. C-peptide concentrations in umbilical cord serum are strongly regulated by the maternal environment(2). We found higher C-peptide levels in newborns of nondiabetic mothers with birth weights at least 3.5 kg than in those with birth weights between 2.0 and 3.5 kg(3). Fetal hyperinsulinism, as in fetuses of diabetic mothers, results in overgrowth; fetal insulin-deficiency, on the other hand, is associated with intrauterine growth retardation (IUGR) in both humans and experimental animals(4,5).
The genes for IGF are expressed in most fetal tissues; IGF-II is expressed more abundantly than IGF-I(6,7). The IGF appear to act in an autocrine and paracrine mode, mainly through the type 1 IGF receptor(8,9). Although the concentrations of both IGF-I and IGF-II in cord serum are correlated with birth weight, the correlation is better for IGF-I(3). Homozygous deletion of the IGF-I gene or deletion of the paternal IGF-II gene (there is genomic imprinting of IGF-II) is associated with IUGR in humans and transgenic mice(8,10).
The bioavailability of the IGF to the receptors is modulated by the IGF binding proteins (IGFBP1-IGFBP6), the genes of which are expressed in various fetal tissues; however, IGFBP1 is produced mainly by the fetal liver(11). Cord serum IGFBP1 concentrations are negatively correlated with birth weight(12), and are markedly higher in newborns with IUGR than in those with normal birth weight(3,13) as well as in IUGR members of twins(2). Experimental IUGR in rats is associated with increased hepatic gene expression and serum levels of IGFBP1(14); conversely, overexpression of the IGFBP1 gene in transgenic mice reduces birth weight by 8-17%(15).
The genes for the IGF, the IGFBP, and the type 1 IGF receptor are also expressed in the placenta and membranes(7,16,17); again, the IGF-II gene is expressed more abundantly than IGF-I(16). The IGFBP1 gene is expressed only in the decidua, but abundantly so(16); concentrations of IGFBP1 in amniotic fluid (AF) are 100- to 1000-fold higher than in adult serum, so that AF is generally used as the source to purify IGFBP1(18).
The diagnosis of IUGR would be greatly improved if biochemical markers could be measured well before IUGR is suspected clinically: maternal serum or AF could be used to this end. In this hospital, 5.1% of women undergo midtrimester amniocentesis for genetic reasons. Hakala-Ala-Pietilä et al.(19) reported that high IGFBP1 levels in AF (i.e. more than 3 multiples of the median) at 15-16 wk gestational age (GA) were more frequent in small for gestational age (SGA) children; maternal serum levels were not predictive, however. Second-trimester AF levels of insulin or C-peptide have been reported to be higher in women with pregestational or (subsequent) gestational diabetes, or with a family history of diabetes(20,21), factors known to be associated with increased birth weight.
In clinical practice, it is not possible to perform all amniocenteses within a short time (e.g. 15-16 wk). A significant number of women undergo amniocentesis between 17 and 20 wk, because of late booking or an abnormal maternal serum α-fetoprotein (AFP) test. The primary aim of this study was to repeat the study of Hakala-Ala-Pietilä et al.(19) in a larger group of patients and in a clinical setting with amniocenteses performed between 14 and 20 wk of pregnancy. To this end, we purified IGFBP1 from AF and developed an RIA. We also measured concentrations of IGF-I, IGF-II, and C-peptide in the same AF samples. These parameters were related to GA at sampling and biometric parameters at birth, including the birth weight percentile distribution.
METHODS
Subjects. Two hundred sixteen women who attended the antenatal clinic, carried a singleton pregnancy, and had an amniocentesis for genetic reasons between 14 and 20 wk of pregnancy entered the study. All patients gave consent for the extra tube of AF for the purpose of the study; the study was approved by the local ethical committee. The parameters recorded at this stage were maternal age at delivery; parity (nulliparous or parous), and GA at amniocentesis. Mean maternal age at confinement was 34.6 y (range, 19-46 y); 126 (60%) were 35 y or older, and 75% were parous. The recorded GA was the best estimate from the last menstrual period or ultrasound performed in the first trimester. The genetic report of each amniocentesis mentioned the karyotype of the fetus and the AF AFP concentration. At this stage, seven patients were excluded from further analysis: one mother with pregestational (type 1) diabetes; four fetuses with an abnormal karyotype (three with Down syndrome and one with 47,XYY) and one fetus with a major congenital malformation detected at amniocentesis (lumbosacral meningomyelocele); and one patient with uncertain GA at amniocentesis (20 wk?). Thus, 209 AF samples were analyzed: 105 fetuses had karyotype 46,XY and 104, 46,XX. At birth, the following parameters were recorded: GA; weight, length, and head circumference of the newborn; placental weight; the occurrence of hypertension or gestational diabetes; and whether the newborn had major congenital abnormalities diagnosed before discharge. Birth weight percentiles were taken from the recent Flemish "SPE-standaarden," derived from approximately 429 000 births in Flanders, Belgium; these percentile charts are available for all newborns together, or for four subgroups according to the parity of the mother (nulliparous or parous) and the sex of the baby(22). The ponderal index was calculated as weight (grams)/length (centimeters)3. Hypertension during pregnancy was classified according to Davey and MacGillivray(23). Eight patients were excluded from the analysis with birth parameters for the following reasons: six patients were delivered elsewhere, and two patients were treated with insulin for gestational diabetes; thus, the birth weight analysis pertains to 201 patients. Nine of these patients were delivered before 37 wk, 15 at 37 wk, 41 at 38 wk, 60 at 39 wk, 44 at 40 wk, 30 at 41 wk, and two at 42 wk. Thirty-four women (17%) had any form of hypertensive disease during the course of pregnancy: five (2.5%) had gestational proteinuric hypertension (preeclampsia), 18 gestational hypertension, eight chronic hypertension, one chronic renal disease, and two unclassified hypertension. Two patients had subtle glucose intolerance, which was untreated: one patient had one abnormal value on oral glucose tolerance test, and another had an abnormal glucose challenge test but had no subsequent oral glucose tolerance test.
Purification of IGFBP1, and development and characteristics of an RIA for IGFBP1. IGFBP1 was purified from an AF sample obtained by amniocentesis from a patient with polyhydramnios, in three steps. 1) An IGF-I affinity column was prepared as described by Baxter et al.(18) by coupling 5 mg of recombinant human (rh) IGF-I (kindly donated by Pharmacia AB, Stockholm, Sweden) to 5 g Affigel 10 (BioRad, Hercules, CA); 250 mL of AF was applied onto the column, which was washed with 0.25 M NaCl and eluted with 0.5 M acetic acid, as described previously(18). In the eluted fractions, the protein concentration was measured by the Lowry method and the binding of radiolabeled IGF-I was measured by competition using an RIA for IGF-I(24), with rhIGF-I as radiolabel and a polyclonal guinea-pig antiserum. 2) The active fractions were lyophilized, redissolved in Tris-HCl 0.025 M, and equilibrated on a Sephadex G-25 M column (PD-10, Pharmacia, Uppsala, Sweden) against the same buffer. The sample was then applied onto a Mono Q HR 5/5 column (Pharmacia) and eluted with a linear 20-mL gradient from 50 to 500 mM NaCl in 25 mM Tris-HCl (pH 7.4); the protein was eluted at approximately 200 mM NaCl. 3) Further purification was obtained using a Superose 12 h 10/30 column (Pharmacia). The purified protein was eluted with a slightly larger elution volume than ovalbumin (Mr, 45 kD); at SDS-PAGE under reducing conditions, a single band with an Mr of 32 kD was obtained by Coomassie blue staining. The IGFBP1 concentration in this standard solution, as measured by an IEMA kit (Medix Biochemica, Kauniainen, Finland) was 90 mg/L; the protein concentration measured by the Lowry method was 134 mg/L. A New Zealand White rabbit was injected intradermally each month with the purified protein; Freund's complete and incomplete adjuvants were used for the primary injections and the boosters, respectively. The antiserum used in the RIA was obtained after three boosters. 125I-labeled IGFBP1 was prepared using the Iodogen method (Pierce, Rockford, IL), with a typical specific activity of 284 mCi/mg. The assay tubes contained 100 µL of standard solution or sample (serum was diluted 1:10 and AF 1:1000 or 1:10 000 in 0.14 M NaCl and 1% HSA, pH 3.0) and 1 mL of antiserum (diluted 1:20 000) in assay buffer (0.1 M phosphate at pH 7.4, 0.01% merthiolate, 0.05 M EDTA, 0.1% BSA, 300 mg/L aprotinin in 0.9% NaCl). After 24 h of incubation at 4°C, 125I-labeled IGFBP1 (approximately 10 000 cpm) was added and incubated overnight. Free and bound fractions were separated by adding 100 µL of secondary antibody-coated cellulose suspension (SacCel, IDS Ltd, Bolton, UK) and incubated for 1 h at 4°C. After centrifugation at 25 rpm for 10 min, the pellet was washed with Tween 20 in 0.9% NaCl. The bound fraction was counted in a gamma-counter. All samples were assayed in duplicate.
The detection limit of the assay is 25 pg per tube. In dilution curves (five further dilutions from 1:1 to 1:20) of serum samples, the overall mean percentage of found over expected IGFBP1 was 99 ± 10% (mean ± SD). The intraassay variation of six serum samples ranged from 2.4 to 4.0% (10 replicate samples for each); the intraassay variation of six AF samples ranged from 3.9 to 5.7% (14 replicate samples for each). The analytical recovery (after adding 5-100 µg of IGFBP1 to three serum samples) was 106 ± 5%. The interassay coefficient of variation of two serum samples with concentrations in the steepest part of the standard curve was 6.2 (n = 15) and 9.7% (n = 14), respectively. IGFBP1 was measured in serum samples from adult men (n = 82), adult women not on oral contraceptives (n = 71), and women on oral contraceptives (n = 58): means ± SD were 9.4 ± 7.3, 13.6 ± 9.7, and 21.6 ± 11.6 µg/L, or 294 ± 228, 424 ± 304, and 675 ± 362 pmol/L (conversion factor, 1 µg/L = 31.25 pmol/L). The values were higher in women (p = 0.003) than in men, and were higher (p < 0.001) in women on oral contraceptives. In nine pregnant women who had a 3-h 100 g oral glucose tolerance test, insulin levels peaked at 1 h (655 ± 267% of the fasting value), whereas IGFBP1 concentrations gradually dropped to 84 ± 10, 63 ± 7, and 55 ± 9% of the fasting value after 1, 2, and 3 h, respectively. This confirms the previously documented down-regulation of serum IGFBP1 by insulin(25).
Other assays. IGF-I, IGF-II, and C-peptide were measured by RIA as previously described(3,24). Before assay, IGF-I was extracted with acid ethanol, and IGF-II was extracted with formic acid-acetone. A dilution curve was performed in an AF sample with high IGF-I or IGF-II concentration: the found over expected values were 99 ± 7% for IGF-I and 102 ± 10% for IGF-II. In the C-peptide RIA, the sample volume was 100 µL of AF instead of 50 µL of serum. AFP was measured by a heterogeneous sandwich magnetic separation assay (Technicon Immuno 1 System, Bayer Corp, Tarrytown, NY).
Statistical analysis. Data analysis was performed using a software program (NCSS, Kaysville, UT). The analysis included one-factor and two-factor ANOVA to evaluate the effects of GA, hypertension, and other variables. When applicable, Scheffé's multiple comparison test was used to detect differences between groups. Correlations were examined by Spearman rank correlation coefficients.
RESULTS
Figures 1–4 show that the AF concentrations of AFP, IGF-I, IGF-II, IGFBP1, and C-peptide were strongly correlated with GA at amniocentesis (p < 0.0001 for all): AFP concentrations were negatively correlated with GA, whereas IGF-I, IGF-II, IGFBP1, and C-peptide concentrations were positively correlated with GA. The AF concentrations of IGF-I, IGF-II, and IGFBP1 were strongly correlated with one another, even after correction for GA at amniocentesis (Table 1). There was a positive correlation between AF C-peptide concentrations and IGF-I, IGF-II, or IGFBP1 concentrations, which disappeared after correcting for GA. AFP concentrations were negatively correlated with IGF-I, IGF-II, IGFBP1, and C-peptide concentrations, which became less significant or NS after correcting for GA.
Box plots of AFP concentrations in AF between 14 and 20 wk of pregnancy. For pictorial clarity, the values of 19 and 20 wk were combined; n of samples for each wk is given in parentheses. The boxes represent the interquartile range (IQR, P25-P75), and the line within the boxes is the median; the bars represent the upper (P75 + 1.5 × IQR) and lower (P25 - 1.5 × IQR) adjacent values. · denotes mild outliers (outside values <3 IQR from the adjacent values. AF AFP levels were correlated with GA (ANOVA, p < 0.0001). Scheffé's multiple-comparison test denotes significant (p < 0.05) differences (a, b, c, d, e, and f) with concentrations at 14, 15, 16, 17, 18, or 19 + 20 wk, respectively.
Box plots of C-peptide concentrations in AF between 14 and 20 wk of pregnancy. For pictorial clarity, the values of 19 and 20 wk have been combined; for n of samples, see Figure 1. The boxes represent the IQR (P25-P75), and the line within the boxes is the median; the bars represent the upper (P75 + 1.5 × IQR) and lower (P25 - 1.5 × IQR) adjacent values. · denotes mild outliers (outside values <3 IQR from the upper adjacent value). AF C-peptide levels were correlated with GA (ANOVA, p < 0.0001). Scheffé's multiple-comparison test denotes significant (p < 0.05) differences (a, b, c, d, e, and f) with concentrations at 14, 15, 16, 17, 18, or 19 + 20 wk, respectively.
There was a negative correlation between AF concentrations of IGF-I, IGF-II, and IGFBP1, and birth weight and placental weight (Table 1). After correcting for GA at amniocentesis and GA at birth, the correlations between the levels of any of these AF factors and birth weight were NS. However, the correlations between AF IGF-I, IGF-II, or IGFBP1 concentrations, and placental weight or the placenta weight to birth weight percentage remained significant.
Subsequently, the birth weights were divided into birth weight percentile classes (Flemish charts): <P10 (SGA infants); P10-P24; P25-P49; P50-P74; P75-P89; and ≥P90 (large for gestational age infants). The frequency distribution (percentage of total) of the 201 newborns in this study was as follows: 8.5% <P10 (expected, 9.9%); 13.4% P10-P24 (expected, 15.0%); 25.9% P25-P49 (expected, 25.0%); 26.4% P50-P74 (expected, 25.0%); 14.9% P75-P89 (expected, 15.0%); and 10.9% ≥ P90 (expected, 10.1%). The frequency distribution of birth weights adjusted for parity and newborn sex was similar (data not shown). For each of the GA weeks at amniocentesis, the values for IGF-I, IGF-II, IGFBP1, and C-peptide were divided into two groups: values ≤P50 and those >P50. Figure 5 shows that the birth weight percentile distribution appears to be comparable for AF IGF-I, IGF-II, C-peptide, and IGFBP1 values ≤P50 versus >P50. The respective birth weights (means ± SD) for newborns with values ≤P50 versus >P50 were 3350 ± 527 (n = 97) versus 3273 ± 602 g (n = 104) for IGF-I; 3350 ± 522 (n = 102) versus 3269 ± 609 g (n = 99) for IGF-II; 3328 ± 595 (n = 101) versus 3292 ± 539 g (n = 100) for IGFBP1; and 3320 ± 575 (n = 117) versus 3291 ± 560 g (n = 83) for C-peptide (all comparisons were NS). Similarly, there was no difference in the birth weight distribution adjusted for parity and newborn sex, for the ≤P50 and >P50 groups (data not shown). Conversely, the AF values of newborns with birth weights <P50 versus ≥P50 were examined for each parameter per GA (the values at 19 and 20 wk were combined in this analysis). None of the comparisons showed significantly different values, except the C-peptide levels at 16 wk (p = 0.04). This was also true for newborns with birth weights <P25 versus ≥P75 (data not shown).
Distribution of birth weight percentile groups (percentage of total number of newborns) in two groups of newborns, with values of IGF-I (left upper panel), IGF-II (right upper panel), IGFBP1 (left lower panel), or C-peptide (right lower panel) that were either ≤P50 (hatched bars) or >P50 (open bars) of the values in AF for each of the 6 wk at amniocentesis (the values of 19 and 20 wk were combined in this analysis). The birth weight percentile groups were derived from Flemish reference charts(22) (see "Methods" and "Results"). Mean birth weights were not different between the two groups (see "Results").
Two-factor ANOVA was performed to evaluate the effects on AF concentrations of IGF-I, IGF-II, IGFBP1, and C-peptide of 1) GA at amniocentesis (the values from 19 and 20 wk were combined in this analysis) and 2) any form of hypertension. The analysis confirmed the effect of GA (p < 0.0001 for all factors), whereas there was no effect of hypertension during pregnancy on AF concentrations of IGF-I and IGFBP1 (p = 0.69 and p = 0.38, respectively). However, there was an effect of hypertension during pregnancy on AF concentrations of IGF-II (p = 0.001; interaction between GA and hypertension, NS) and C-peptide (p = 0.002; interaction, NS): both IGF-II and C-peptide concentrations in AF were higher in women with any form of hypertension during pregnancy than in women who remained normotensive [177 ± 12 versus 127 ± 5 nM (means ± SEM), respectively, for IGF-II; 0.048 ± 0.002 versus 0.038 ± 0.001 nM, respectively, for C-peptide]. There was a scatter in the AF values of IGF-I, IGF-II, and IGFBP1 in the five women who subsequently had preeclampsia, e.g. their IGFBP1 concentrations were <P5, P65-P70, P70-P75, P85-P90, and >P95 of the values measured at the GA (data not shown).
Two-factor ANOVA was used to examine the effects on birth weight and placental weight of 1) GA at birth and 2) any form of hypertension during pregnancy. (In this analysis, all births >37 wk were classified as 36 wk and the values of 41 and 42 wk were combined as 41 wk.) The analysis showed a positive effect of GA on birth weight and placental weight (p < 0.0001), but a negative effect on placenta weight to birth weight percentage (p < 0.0001); hypertension had no effect on birth weight (p = 0.61; interaction with GA, p = 0.04), but decreased placental weight (p = 0.015; interaction, NS) and the placenta weight to birth weight percentage (p = 0.005; interaction, p = 0.007).
We examined whether the correlation of AF concentrations of IGF-I, IGF-II, and IGFBP1 with placental weight held true for normotensive women (n = 167) by Spearman correlations. Placental weight was negatively correlated with AF concentrations IGF-I (r = -0.17; p = 0.03), IGF-II (r = -0.23; p = 0.004), and IGFBP1 (r = -0.22; p = 0.005) in normotensive women; after correction for GA at amniocentesis and at birth, the negative correlation was still significant or there was a trend (r with IGF-I = -0.14; p = 0.09; r with IGF-II = -0.20; p = 0.01; r with IGFBP1 = -0.20; p = 0.01). This was also true for placenta weight to birth weight percentage corrected for GA: r with IGF-I = -0.14; p = 0.08; r with IGF-II = -0.26; p = 0.0009; r with IGFBP1 = -0.29; p = 0.0003. The correlations in the hypertensive group were NS.
Finally, two-factor ANOVA was used to examine the effects on placental weight and placenta weight to birth weight percentage of 1) GA at birth and 2) AF concentrations of IGF-I, IGF-II, and IGFBP1 (≤P50 versus >P50). The effect of GA was predominant (p < 0.001 for all analyses); however, the placenta weight to birth weight percentage was also determined by the AF IGFBP1 concentrations (Fig. 6).
Placental weight to birth weight percentage with increasing GA in two groups, with AF concentrations of IGFBP1 that were either ≤P50 or >P50 for each of the weeks at amniocentesis. In this analysis, the values of AF IGFBP1 at 19 and 20 wk were combined; births at less than 37 wk were combined as 36 wk, and births at 41 and 42 wk were combined as 41 wk. Two-factor ANOVA showed that both GA at birth (p < 0.0001) and AF IGFBP1 concentrations (≤P50 vs >P50) (p = 0.0003) determined the placental weight to birth weight percentage.
DISCUSSION
Several observations were made in this study. First, there is a gradual increase in the AF concentrations of IGF-I (5-fold increase of the median value), IGF-II (3-fold increase), IGFBP1 (8-fold), and C-peptide (2.5-fold) between 14 and 19-20 wk of pregnancy. This is not in complete agreement with data reported by Chard et al.(26), who found a 20-fold increase in the median AF IGF-II concentration between 10 and 20 wk; AF IGFBP1 levels rose dramatically between 9 and 16 wk (4000-fold increase in the median value), but were lower between 17 and 20 wk than at 16 wk(27). In a recent study, the median AF insulin level (measured from 16 wk onward) increased slightly (less than 2-fold) until about 31 wk, with a small decline thereafter(28). Unlike these growth-modulating peptides, we confirmed that AF AFP concentrations drop during the second trimester(29). The gene encoding this plasma protein is expressed in the fetal liver, and is evolutionary linked to the albumin gene(30). The second observation of this study is that the concentrations of IGF-I, IGF-II, and IGFBP1 in second-trimester AF are strongly correlated, even after correction for GA. This is interesting, because IGF-I and IGF-II are secreted by the placenta and membranes, whereas IGFBP1 is secreted by the decidua(16); apparently, the synthesis of these growth modulators is regulated by common, possibly genetic, factors.
Third, the concentrations of IGF-I, IGF-II, and IGFBP1 in second-trimester AF were markedly higher than in third-trimester cord serum or adult serum samples, previously measured with the same assays(3). Thus, mean AF IGF-I concentrations were between 50 and 189 nM, compared with 8.8 ± 0.5 nM (mean ± SEM) in cord sera at 39 wk, and 38 ± 1 nM in 227 adult samples. Mean IGF-II concentrations in AF were between 59 and 212 nM, compared with 56 ± 1 nM in cord sera at 41 wk, and 100 ± 1 nM in adults. Mean IGFBP1 concentrations were between 265 and 1614 nM, i.e. 400-5000-fold higher than in adult sera (see "Methods"). By contrast, C-peptide concentrations in AF (means between 0.026 and 0.055 nM) were about 10-fold lower than in cord sera (means between 0.30 and 0.37 nM) and adult samples (0.50 ± 0.03 nM).
Fourth, this study demonstrates that second-trimester AF concentrations of IGF-I, IGF-II, IGFBP1, and C-peptide do not predict birth weight, IUGR, or adiposity (as reflected by the ponderal index). This was the major aim of the study, and our results are in apparent disagreement with an earlier study with amniocenteses performed at 15-16 wk(19). In that study, very high AF IGFBP1 concentrations (>P90 or more than 3 multiples of the median) were more frequently found in SGA newborns, but no inverse correlation with future birth weight was reported. In retrospect, our negative result is not unexpected. Fetal weight gain is concentrated in the third trimester: median birth weight at 28 wk is 32% of that at 40 wk(22). It is dependent on the maternal metabolic environment (primarily plasma glucose levels), which is the main regulator of fetal insulin levels(2), and on the uteroplacental blood flow rate, which regulates fetal IGF-I and IGFBP1 gene expression and may also regulate insulin secretion(14,31). Future studies should assess whether various isoforms of AF IGFBP1 (phosphorylated versus nonphosphorylated) can predict birth weight(32).
A fifth and novel finding of this study is that placenta weight and the placenta weight to birth weight percentage were negatively correlated with AF concentrations of IGFBP1 and IGF-II, and IGF-I to a lesser extent. These negative correlations remained after corrections for GA and were also present in normotensive pregnancies. This excluded the possibility that the correlation was somehow explained by hypertension in pregnancy (AF IGF-II was indeed higher in hypertensive pregnancies), which we confirmed to be associated with lower placenta weight. Although placenta weight is a crude measure of placental growth and aging (in this study, mean placenta weight was maximal at 39 wk), the data would suggest that placental development is negatively affected by these growth-modulating factors. Little is known about the regulation of placental growth and degeneration. It is possible that high decidual expression of the IGFBP1 gene is associated with shallow placental invasion, because serum IGFBP1 levels were found to be higher in mothers with severe preeclampsia(33). However, we found no consistent change in the AF concentrations of IGFBP1 in women who later had preeclampsia; de Groot et al.(34) reported that IGFBP1 concentrations in maternal serum were actually lower in women who later had preeclampsia, which they attributed to an abnormal decidual stroma function with onset before clinical manifestation of preeclampsia. It would be interesting to study placental development in mice with overexpression of the IGFBP1 gene(15). The negative correlation of AF IGF-II (and IGF-I) with placenta weight is more difficult to explain, given the fact that the IGF are produced by the placenta, and that IGF-II is believed to stimulate cytotrophoblast proliferation and trophoblast invasion(16,17). In mice, placental growth is not regulated by IGF-I, whereas IGF-II stimulates placental growth via an unknown receptor that is not the type 1 IGF receptor nor the type 2 IGF (mannose-6-phosphate) receptor(9). Clearly, more research is warranted on the regulation of placental development by the IGF and IGFBP1.
Interestingly, epidemiologic studies have shown that a high placenta weight to birth weight percentage is associated with a higher risk of glucose intolerance, elevated blood pressure, and death from cardiovascular disease in adulthood(35,36). Thus, low production of IGF and IGFBP1 by maternal tissues, placenta and membranes, or the fetus may be causally related to the induction of insulin resistance in the fetus that appears to explain the above clinical manifestations.
The contributions of the maternal tissues, placenta and membranes, and the fetus to the volume as well as the composition of AF in the second trimester are not well understood. Martina et al.(32) produced suggestive evidence that high concentrations of IGFBP1 in second-trimester AF are largely derived from the maternal decidua, whereas the fetal contribution is biologically insignificant at this stage of pregnancy. Insulin (or C-peptide) in midtrimester AF, on the other hand, is derived from the fetus, because there is no transplacental transport: immunoreactive insulin is present in the human fetal pancreas as early as 11 wk(1). In addition, the placenta expresses insulin-related genes, the significance of which needs further study(37). The IGF in second-trimester AF are presumably derived in large part from secretion of placenta and membranes(7,16,17), whereas the fetal contribution should be small at this time given the low fetal serum levels, especially those of IGF-I; further studies must confirm this hypothesis. One would assume that high concentrations of IGF and IGFBP1 in AF affect fetal development [e.g. the fetus swallows AF from 12 wk onward(38)]. The regulation of fetal development (and its long-term consequences) by the maternal-fetal interface is an exciting area for future research.
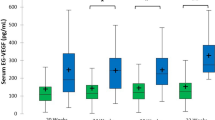
Box plots of IGF-I and IGF-II concentrations in AF between 14 and 20 wk of pregnancy. For pictorial clarity, the values of 19 and 20 wk were combined; for n of samples, see Figure 1. The boxes represent the IQR (P25-P75), and the line within the boxes is the median; the bars represent the upper (P75 + 1.5 × IQR) and lower (P25 - 1.5 × IQR) adjacent values. · denotes mild outliers (outside values <3 IQR from the upper adjacent values) and ▴ denotes severe outliers (>3 IQR). Both IGF-I and IGF-II levels in AF were correlated with GA (ANOVA, p < 0.0001). Scheffé's multiple-comparison test denotes significant (p < 0.05) differences (a, b, c, d, e, and f) with concentrations at 14, 15, 16, 17, 18, or 19 + 20 wk, respectively.
Box plots of IGFBP1 concentrations in AF between 14 and 20 wk of pregnancy. For pictorial clarity, the values of 19 and 20 wk were combined; for n of samples, see Figure 1. The boxes represent the IQR (P25-P75), and the line within the boxes is the median; the bars represent the upper (P75 + 1.5 × IQR) and lower (P25 - 1.5 × IQR) adjacent values. ▴ denotes a severe outlier (outside value >3 IQR from the upper adjacent value). AF IGFBP1 levels were correlated with GA (ANOVA, p < 0.0001). Scheffé's multiple-comparison test denotes significant (p < 0.05) differences (a, b, c, d, e, and f) with concentrations at 14, 15, 16, 17, 18, or 19 + 20 wk, respectively.
Abbreviations
- AF:
-
amniotic fluid
- AFP:
-
α-fetoprotein
- GA:
-
gestational age
- IGFBP1:
-
IGF binding protein 1
- IUGR:
-
intrauterine growth retardation
- SGA:
-
small for gestational age
References
Rastogi GK, Letarte J, Fraser TR 1970 Immunoreactive insulin content of 203 pancreases from foetuses of healthy mothers. Diabetologia 6: 445–446
Verhaeghe J, Loos R, Vlietinck R, Van Herck E, van Bree R, De Schutter A-M 1996 C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in cord serum of twins: genetic versus environmental regulation. Am J Obstet Gynecol 175: 1180–1188
Verhaeghe J, Van Bree R, Van Herck E, Laureys J, Bouillon R, Van Assche FA 1993 C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in umbilical cord serum: correlations with birth weight. Am J Obstet Gynecol 169: 89–97
Kruger C, Dorr HG, von Muhlendahl KE, Herkenhoff H 1997 Neonatal diabetes and intra-uterine growth retardation. Eur J Pediatr 156: 1–2
Fowden AL 1993 Insulin deficiency: effects on fetal growth and development. J Paediatr Child Health 29: 6–11
Han VKM, Lund PK, Lee DC, D'Ercole AJ 1988 Expression of somatomedin/insulin-like growth factor messenger ribonucleic acids in the human fetus: identification, characterization, and tissue distribution. J Clin Endocrinol Metab 66: 422–429
Birnbacher R, Amann G, Breitschopf H, Lassmann H, Suchanek G, Heinz-Erian H 1998 Cellular localization of insulin-like growth factor II mRNA in the human fetus and the placenta: detection with a digoxigenin-labeled cRNA probe and immunocytochemistry. Pediatr Res 43: 614–620
Liu J-P, Baker J, Perkins AS, Robertson EJ, Efstratiadis A 1993 Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igflr). Cell 75: 59–72
Baker J, Liu J-P, Robertson EJ, Efstratiadis A 1993 Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75: 73–82
Woods KA, Camacho-Hübner C, Savage MO, Clark AJL 1996 Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335: 1363–1367
Han VKM, Matsell DG, Delhanty PJD, Hill DJ, Shimasaki S, Nygard K 1996 IGF-binding protein mRNAs in the human fetus: tissue and cellular distribution of developmental expression. Horm Res 45: 160–166
Wang HS, Lim J, English J, Irvine L, Chard T 1991 The concentrations of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. J Endocrinol 129: 459–464
Giudice LC, de Zegher F, Gargosky SE, Dsupin BA, de las Fuentes L, Crystal RA, Hintz RL, Rosenfeld RG 1995 Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab 80: 1548–1555
Unterman T, Lascon R, Gotway MB, Oehler D, Gounis A, Simmons RA, Ogata ES 1990 Circulating levels of insulin-like growth factor binding protein-1 (IGFBP-1) and hepatic mRNA are increased in the small for gestational age (SGA) fetal rat. Endocrinology 127: 2035–2037
Rajkumar K, Barron D, Lewitt MS, Murphy LJ 1995 Growth retardation and hyperglycemia in insulin-like growth factor binding protein-1 transgenic mice. Endocrinology 136: 4029–4034
Han VKM, Bassett N, Walton J, Challis JRG 1996 The expression of insulin-like growth factor (IGF) and IGF-binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF-IGFBP interactions at the fetomaternal interface. J Clin Endocrinol Metab 81: 2680–2693
Thomsen BM, Clausen HV, Larsen LG, Nürnberg L, Ottesen B, Thomsen HK 1997 Patterns in expression of insulin-like growth factor-II and of proliferative activity in the normal human first and third trimester placenta demonstrated by non-isotopic in situ hybridization and immunohistochemical staining for MIB-1. Placenta 18: 145–154
Baxter RC, Martin JL, Wood MH 1987 Two immunoreactive binding proteins for insulin-like growth factors in human amniotic fluid: relationship to fetal maturity. J Clin Endocrinol Metab 65: 423–431
Hakala-Ala-Pietilä TH, Hakala-Ala-Pietila TH, Koistinen RA, Salonen RK, Seppälä MT 1993 Elevated second-trimester amniotic fluid concentration of insulin-like growth factor binding protein-1 in fetal growth retardation. Am J Obstet Gynecol 169: 35–39
Fallucca F, Sciullo E, Napoli A, Cardellini G, Maldonato A 1995 Amniotic fluid insulin and C peptide levels in diabetic and nondiabetic women during early pregnancy. J Clin Endocrinol Metab 80: 137–139
Carpenter MW, Canick JA, Star J, Carr SR, Burke ME, Shahinian K 1996 Fetal hyperinsulinism at 14-20 weeks and subsequent gestational diabetes. Obstet Gynecol 87: 89–93
Devlieger H, Martens G, Bekaert A, Eeckels R, Vlietinck R 2 . Standaarden van geboortegewicht-voor-zwangerschapsduur voor de Vlaamse boreling. In: Bekaert A, Martens G, Devlieger H (eds) Perinatale Activiteiten in Vlaanderen 1996 (Studiecentrum voor Perinatale Epidemiologie), Brussel, pp 94–116
Davey DA, MacGillivray I 1988 The classification and definition of hypertensive disorders of pregnancy. Am J Obstet Gynecol 158: 892–898
Verhaeghe J, Suiker AMH, Visser WJ, Van Herck E, Van Bree R, Bouillon R 1992 The effects of systemic insulin, insulin-like growth factor I and growth hormone on bone growth and turnover in spontaneously diabetic BB rats. J Endocrinol 134: 485–492
Suikkari AM, Koivisto VA, Koistinen R, Seppälä M, Yki-Järvinen H 1989 Dose-response characteristics for suppression of low molecular weight plasma insulin-like growth factor-binding protein by insulin. J Clin Endocrinol Metab 68: 135–140
Chard T, Blum WF, Brunjes J, Campbell DJ, Wathen NC 1994 Levels of insulin-like growth factor-binding protein-2 and insulin-like growth factor-II in maternal serum, amniotic fluid and extraembryonic coelomic fluid at 9-20 wk of pregnancy. J Endocrinol 142: 379–383
Wathen NC, Egembah S, Campbell DJ, Farkas A, Chard T 1993 Levels of insulin-like growth factor-binding protein-I increase rapidly in amniotic fluid from 11 to 16 weeks of pregnancy. J Endocrinol 137:R1–R4
Weiss PAM, Kainer F, Haeusler M, Pürstner P, Haas J 1998 Amniotic fluid insulin levels in nondiabetic women: an update. Arch Gynecol Obstet 262: 81–86
Weiss PAM, Pürstner P, Lichtenegger W, Winter R 1978 Alpha-fetoprotein content of amniotic fluid in normal and abnormal pregnancies. Obstet Gynecol 51: 582–585
Camper SA, Godbout R, Tilghman SM 1989 The developmental regulation of albumin and α-fetoprotein gene expression. Prog Nucleic Acid Res Mol Biol 36: 131–143
Straus DS, Ooi GT, Orlowski CC, Rechler MM 1991 Expression of the genes for insulin-like growth factor-I (IGF-I), IGF-II, and IGF-binding proteins-1 and -2 in fetal rat under conditions of intrauterine growth retardation caused by maternal fasting. Endocrinology 128: 518–525
Martina NA, Kim E, Chitkara U, Wathen NC, Chard T, Giudice LC 1997 Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J Clin Endocrinol Metab 82: 1894–1898
Giudice LC, Martina NA, Crystal RA, Tazuke S, Druzin M 1997 Insulin-like growth factor binding protein-1 at the maternal-fetal interface and insulin-like growth factor-I, insulin-like growth factor-II, and insulin-like growth factor binding protein-1 in the circulation of women with severe preeclampsia. Am J Obstet Gynecol 176: 751–758
de Groot CJM, O'Brien TJ, Taylor RN 1996 Biochemical evidence of impaired trophoblastic invasion of decidual stroma in women destined to have preeclampsia. Am J Obstet Gynecol 175: 24–29
Phipps K, Barker DJP, Hales CN, Fall CHD, Osmond C, Clark PMS 1993 Fetal growth and impaired glucose tolerance in men and women. Diabetologia 36: 225–228
Barker DJP, Osmond C, Simmonds SJ, Wield GA 1993 The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ 306: 422–426
Liu K-S, Wang C-Y, Mills N, Gyves M, Ilan J 1985 Insulin-related genes expressed in human placenta from normal and diabetic pregnancies. Proc Natl Acad Sci USA 82: 3868–3870
de Vries JIP, Visser GHA, Prechtl HFR 1982 The emergence of fetal behaviour. I. Qualitative aspects. Early Hum Dev 7: 301–322
Acknowledgements
The authors thank Prof. W. Heyns for his advice and help with the IGFBP1 purification, Prof. F.A. Van Assche for supervising this project, and Prof. R. Bouillon for supervising the amniotic fluid measurements. We also thank Drs. K. Devriendt and J. Billen for their help with the data collection. The ultrasound nursing staff kindly assisted with the sample collection.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Verhaeghe, J., Coopmans, W., van Herck, E. et al. IGF-I, IGF-II, IGF Binding Protein 1, and C-Peptide in Second Trimester Amniotic Fluid Are Dependent on Gestational Age but Do Not Predict Weight at Birth. Pediatr Res 46, 101–108 (1999). https://doi.org/10.1203/00006450-199907000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199907000-00017
This article is cited by
-
Markers for cardiovascular disease in monozygotic twins discordant for the use of third-generation oral contraceptives
Journal of Human Hypertension (2003)
-
Tolerance of a Sterile Isotonic Electrolyte Solution Containing Select Recombinant Growth Factors in Neonates Recovering From Necrotizing Enterocolitis
Journal of Perinatology (2003)