Abstract
Rabbit pups were delivered by cesarean section 1 or 2 d before term, or vaginally around term, and then reared in room air or exposed to intermittent or continuous hyperoxia (>85%) for up to 9 d. Pups were killed at different ages, and lung hyaluronan (HA; µg/g of dry lung weight) and lung water content, measured as wet/dry lung weight, were determined. Compared with the day of birth, the lung HA concentration did not change significantly on succeeding days in pups kept in air delivered 2 d (-2 d) or 1 d (-1 d) before term, whereas the water content decreased significantly. Continuous exposure to hyperoxia resulted in a significantly raised lung HA concentration 6 d postterm in both -2 d and -1 d pups, and intermittent exposure to hyperoxia resulted in a significantly raised HA concentration 6 d postterm in -1 d pups, compared with the groups exposed to room air. These increases were accompanied by significantly elevated wet/dry lung weight ratios. Microscopic examination revealed significantly increased HA staining scores in alveoli, arterioles, and bronchioli in both hyperoxia-exposed groups of -2 d pups 6 d postterm, and nonsignificantly higher scores in -1 d and vaginally delivered pups of comparable age, compared with the scores at birth. The results indicate that oxygen exposure neonatally may result in an increase in lung HA accompanied by an increase in lung water content. The increase in lung HA concentration in our study may be an affect of oxygen free radicals or oxygen-induced stimulation of inflammatory mediators.
Similar content being viewed by others
Main
During fetal life the airways are filled with fluid. Before normal birth, the secretion of fluid by the lung epithelium begins to diminish even before the onset of labor (1). At birth the secretion ceases, and absorption of this fluid is dramatically increased (2). Rabbit pups and lambs delivered by cesarean section before labor show a higher lung water content than those delivered after labor, either vaginally or by cesarean section (3); and infants delivered by elective cesarean section before the onset of labor have a higher incidence of respiratory disorders than infants delivered vaginally (4).
After preterm birth, the balance between secretion and absorption in the airways may be disturbed, resulting in interstitial edema. This edema is known to disturb the alveolar gas exchange by obstructing diffusion and probably by increasing ventilation/perfusion mismatching. Treatment often includes prolonged oxygen supplementation and/or ventilatory support (5), both of which per se may increase the tendency to interstitial edema (6).
Hyperoxia, as well as reoxygenation episodes (7), may also, through the production of free oxygen radicals, cause interstitial edema, with consequent repeated obstruction of gas exchange (8) and lung structural damage (9). The water content of the pulmonary interstitium may also be influenced by the concentrations of macromolecules. HA is a large glycosaminoglycan polymer of hyalobiuronic acid, a disaccharide consisting of glucuronic acid and N-acetylglucosamine, with a molecular weight in tissues of 1-10 MD (10). It forms long unbranched chains with a random coil structure and a high capacity to immobilize water. The concentration of HA in different tissues varies widely. It appears in abundance in tissues during development or repair, and during inflammation. It is believed to modify the behavior of infiltrating and resident cells by environment modification and through direct action on specific cellular receptors (10).
In an earlier study of the HA and water content in lungs from rabbit pups in the perinatal period (26), we found that the HA concentration decreased from a high level 6 d before term, to a minimum 1 d before term, and then increased during the first days of life after term birth. The water content was high before term, and then decreased during the first days of life.
In patients with inflammatory diseases of the lungs (11,12) and in experimental alveolitis (13,14), increased HA concentrations have been demonstrated both in interstitial lung tissue and in bronchoalveolar lavage fluid. Parallel to these observations, an increased number of inflammatory cells and an increased lung water content have been noted.
We hypothesized that HA may be an important determining factor for the interstitial water content in acute neonatal lung disease and in the development of bronchopulmonary dysplasia that sometimes results from this disease. The aim of the present work was to study the influence of postnatal age and exposure to hyperoxia on the lung concentration of HA and the lung water content in preterm and term rabbit pups. Two modes of hyperoxic exposure, intermittent and continuous, were investigated based on evidence (15) that hypoxia-reoxygenation can cause more lung damage, and therefore might result in higher HA concentrations, than continuous hyperoxic exposure. Because the activity of lung antioxidant enzymes, and the ability to increase this activity in response to hyperoxic exposure, increases during late gestation (16–18), we also hypothesized that preterm animals would be more affected by hyperoxia than those born at term. Preliminary results from this study have been presented earlier (19–23).
METHODS
New Zealand White rabbits were studied. They have a normal gestation period of 31 d. Female rabbits were inseminated so that the gestational age at birth could be determined. Two groups of pups were delivered by cesarean section 1 or 2 d before term, respectively, whereas pups in a third group were born spontaneously around term. Spontaneous birth occurred at a mean gestational age of 30.7 ± 0.94 (SD) days (range, 29-34 d). All pups were subsequently kept in incubators at 32-34°C either in room air or with continuous or intermittent exposure to hyperoxia. The pups in the continuous-hyperoxia groups received hyperoxic air, 3 L/min, from an oxygen concentrator (Zefir 1, R Liquide, France), and repeated measurements (Hudson Oxygen Monitor, Omeda, V:a Frölunda, Sweden) showed ambient oxygen concentrations of ≥85% at all times. The pups in the intermittent hyperoxia groups received oxygen (AGA, Lidingö, Sweden), 2 L/min, in repeated 20-min cycles, with 20-min intervals. The ambient oxygen concentration in the incubator reached 85% 8 min after the start of oxygen supplementation, and it was ≥85% for the rest of the supplemental period; 7 min after the oxygen supplementation was discontinued, it had returned to 21%. Carbon dioxide was removed by soda lime absorption. The pups were fed standard infant formula twice daily by gavage. The pups were killed by an injected overdose of thiopental sodium at different ages up to 8 d postterm. The lungs were removed en bloc immediately after death. Pups that died unscheduled were excluded from further analysis.
The right lung was fixed in formalin buffer with microwave heating, according to the method described by Hellström et al. (24). From the five lungs in each group that had a concentration of HA/dry lung weight closest to the mean concentration in that group, 5-6 µm thick cryostat sections were taken and stained with hematoxylin and eosin, and labeled in situ for HA with a biotinylated HA-binding protein according to the method of Ripellino et al. (25) as described earlier (26). The intensity of the HA staining of visceral pleura, arterioles, bronchioli, and the alveolar interstitial space was semiquantified on a 0 to 3 scale, as described previously (26), by one author (L.E.) before obtaining knowledge of background data.
The left lung was weighed and frozen and then freeze-dried for 72 h. The dried lung was reweighed, and the water content was calculated as the W/D ratio. The dried lungs were then digested with Pronase (protease P-5147, Streptomyces griseus, Sigma Chemical Co., St. Louis) in a 2.5 U/mL buffer solution (0.05 M Tris, 0.01 M CaCl2, pH 7.2) at 55°C overnight (>16 h). One unit of enzyme was added for each 10 mg of dry lung material. The digestion was terminated by heating to 100°C for 10 min in a water bath. The content of HA was determined with a radiometric assay kit (HA 50, Pharmacia, Uppsala, Sweden) (27), and the HA concentration was calculated in relation to the dry lung weight and body weight.
For analysis, the pups were grouped according to 1) mode of delivery, 2) day of birth, and 3) day of death. All ages are expressed as the whole number of completed gestational days in relation to term (= 31 d of gestation).
Data are presented as means ± SEM. Statistical analysis of continuous variables was performed with one-way ANOVA, followed by either the Bonferroni (equal variance) or the Tamhane T2 (unequal variance) post hoc test. Differences between differently treated groups were evaluated for significance with a two-tailed independent sample t test. The semi-quantitative staining scores and mortality rates were analyzed by the Kruskal-Wallis H test. Significant changes between different groups were evaluated by the Mann-Whitney U test. Covariance analysis was performed with the Pearson correlation. For all statistical analyses the software SPSS version 7.0-7.5 (SPSS, Chicago, IL) was used. In all tests p < 0.05 was considered significant. The work was approved by the Uppsala University Animal Trials Ethics Committee (project numbers C35/90 and C80/91).
RESULTS
Lung HA and W/D in Pups Kept in Room Air
The results are shown in Table 1.
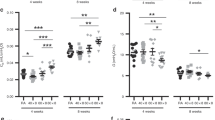
Pups delivered by cesarean section 1 or 2 d before term (-1 and -2 d pups). No significant differences in lung HA concentrations were found between the groups of pups of different postterm age. All the W/D ratios at term onward were significantly lower than the ratio at birth (p < 0.001) (Figs. 1 and 2).
HA/dry lung weight (µg/g) and W/D ratios in rabbit pups delivered 2 d before term by cesarean section and kept in room air (○) or exposed to hyperoxia intermittently (▴) or continuously (▪). Statistical significance: **pups exposed to continuous hyperoxia compared with pups kept in air (p = 0.001); *pups exposed to continuous hyperoxia compared with pups kept in air (p = 0.011) and compared with those exposed to intermittent hyperoxia (p = 0.031).
HA/dry lung weight (µg/g) and W/D ratios in rabbit pups delivered 1 d before term by cesarean section and kept in room air (○) or exposed to hyperoxia intermittently (▴) or continuously (▪). Statistical significance: **pups exposed to continuous hyperoxia compared with pups kept in air (p = 0.001); pups exposed to intermittent hyperoxia compared with pups kept in air (p = 0.009); *pups exposed to continuous hyperoxia compared with pups kept in air (p = 0.045); pups exposed to intermittent hyperoxia compared with pups kept in air (p = 0.024).
Pups delivered spontaneously around term. No significant differences in lung HA concentrations were found between the groups of pups of different postterm age. All W/D ratios from a postterm age of 2-8 d were significantly lower than the ratio 1 d preterm (p = 0.005, p = 0.046, p = 0.011, p = 0.005) (Fig. 3).
Lung HA and W/D in Pups Exposed to Hyperoxia
The results are shown in Table 1. Except for the W/D ratios 4 d postterm in pups born by cesarean section 2 d before term (see below), no significant difference in HA concentration or W/D ratio was found when the groups of pups exposed to the two different modes of hyperoxia were compared.
Pups delivered by cesarean section 2 d before term. A significantly (p = 0.001) higher HA concentration/dry lung weight was found in pups exposed to continuous, but not to intermittent, hyperoxia than in pups kept in room air 6 d postterm. Four days postterm the W/D ratio in the pups exposed to continuous hyperoxia was significantly higher than the ratio in pups kept in room air (p = 0.011), and in pups exposed to intermittent hyperoxia (p = 0.031) (Fig. 1). When pups exposed to both modes of hyperoxia were pooled, they together displayed a significantly higher mean HA concentration than the pups kept in air (p = 0.001) (data not shown).
Pups delivered by cesarean section 1 d before term. Exposure to hyperoxia, both continuous and intermittent, resulted in significantly higher HA/dry lung weight concentrations 6 d postterm than the corresponding concentration in pups kept in room air (p = 0.001, p = 0.009). Concomitantly, significantly higher W/D ratios (p = 0.045, p = 0.024), were noted in comparison with pups kept in air (Fig. 2). When pups exposed to both modes of hyperoxia were pooled, they together displayed a significantly higher mean HA concentration than did the pups kept in air (p = 0.004) (data not shown).
Pups delivered spontaneously around term. Pups delivered spontaneously around term and exposed to continuous or intermittent hyperoxia had nonsignificantly higher HA/dry lung weight concentrations 6 d after term than the pups kept in room air. When pups exposed to both modes of hyperoxia were pooled, they together displayed a significantly higher mean HA concentration than did the pups kept in air (p = 0.042). Four days postterm, the pups exposed to intermittent hyperoxia showed a significantly (p = 0.017) higher W/D ratio than the pups kept in air (Fig. 3).
When pups born spontaneously and killed 4 and 6 d postterm were pooled, highly significant differences were found. Pups exposed to continuous or intermittent hyperoxia showed significantly higher HA/dry lung weight concentrations than did the pups kept in room air (p < 0.001, p = 0.001). The W/D ratios were also significantly higher than those in pups breathing room air (p = 0.003, p < 0.001) (data not shown).
Microscopic Lung Examination
The results are shown in Table 2. Photomicrographs are shown in Figure 4. In pups delivered by cesarean section 2 d before term, the semiquantitative HA staining scores for alveoli, arterioles, and bronchioli in the group exposed to intermittent hyperoxia were significantly higher 6 d postterm than at birth (p = 0.014; p = 0.014; p = 0.014). In the group exposed to continuous hyperoxia only the alveolar score was significantly higher 6 d postterm than at birth (p = 0.015), whereas staining scores for arterioles and bronchioli were higher, with a borderline significance, than those at birth (p = 0.050; p = 0.050, respectively).
Photomicrographs of sections from lungs of pups delivered by cesarean section 2 d before term, stained with hematoxylin and eosin and with biotinylated HA-binding protein (magnification, ×200). (A) Lung from pup killed immediately after delivery. The lung is only partially aerated and displays thick interstitial regions with only slight HA staining. Only the vascular walls show more intense HA staining. (B) Lung from pup kept in room air, killed 6 d postterm. The lung is aerated and shows thin interstitial regions with some HA staining. The vascular walls display more intense HA staining. (C) Lung from pup exposed intermittently to hyperoxia, killed 6 d postterm. The lung is aerated and shows thin interstitial regions with fairly intense HA staining. (D) Lung from pup exposed continuously to hyperoxia, killed 6 d postterm. The lung is aerated and shows thin interstitial regions with intense HA staining. The vascular walls show even more intense HA staining.
In pups delivered by cesarean section 1 d before term, all groups showed increased staining of alveoli 6 d postterm compared with the scores at birth, but no significant differences in semiquantitative HA staining scores were found. In pups delivered spontaneously around term, there were minor increases in staining of alveoli in all groups 4 and 6 d (pooled) postterm, compared with the scores at birth, but there were no significant differences in semiquantitative HA staining scores.
Birth Weight and Weight Increase
Data are not shown. In pups kept in room air, there was no significant difference in birth weight between the groups of pups delivered at the same gestational age and killed at different postnatal ages.
The mean birth weights of the -2 d pups exposed to intermittent hyperoxia and killed 2 and 4 d postterm were significantly different from the birth weights of the corresponding groups kept in room air (p = 0.046, p = 0.022). Pups kept in air had a significantly smaller weight increase 2 d postterm than did pups exposed to both modes of hyperoxia (p = 0.014, p = 0.043), and 6 d postterm than did pups exposed to intermittent hyperoxia (p = 0.025).
The birth weights of the -1 d pups did not differ significantly between the postnatal age or treatment groups. Pups exposed to intermittent hyperoxia had a significantly greater weight increase than pups kept in air at 6 d postterm (p = 0.033). The birth weight or weight increase of the pups born spontaneously around term did not differ significantly between the postnatal age or treatment groups.
Mortality
Inadvertent pup death resulted from esophageal perforation during feeding, from infection, and from unknown causes. The mortality was higher in pups born at a gestation of -2 d or -1 d than in pups born at term. The overall mortality was 35% (Table 3). Both the gestational age at birth and the mode of treatment affected the mortality rate. Among -2 d and -1 d pups there was no significant difference in mortality between the groups exposed to intermittent or continuous hyperoxia and the groups kept in air. In pups born spontaneously around term, the mortality among pups kept in room air was significantly (p < 0.001) lower than that among pups exposed to either mode of hyperoxia.
Covariation between HA and Lung Water
Only a weak correlation between lung HA concentration and lung water content was found. Regression analysis resulted in r = 0.166 and an adjusted r2 of 0.025 for all pups, and r = 0.252 and an adjusted r2 of 0.058 for all pups exposed to hyperoxia.
DISCUSSION
The main finding in this study is that exposure to hyperoxia in the neonatal period in both term and preterm rabbit pups can result in an increase in the lung HA concentration, parallel to an increase in the lung water content. The largest effect was seen in the pups born 1 d before term. In consideration of the lower activity of lung antioxidant enzymes in preterm animals (16–18), we had expected that exposure to hyperoxia would have caused an even more pronounced increase in the lung HA concentration and water content in the pups born 2 d before term, than in pups born closer to term or at term. The observed lower than expected increase in lung HA and lung water content might be a result of the higher inadvertent mortality rate in -2 d pups (see Table 3). In addition, a high lung HA concentration at birth might offer some protection against oxygen injury, as observed by other investigators (28,29). In the present study intermittent exposure to hyperoxia did not lead to a more pronounced increase in lung HA concentration than continuous exposure to hyperoxia. With exposure to hyperoxia alternating with normoxia, there will probably be no periods of hypoxia. Hypoxic periods followed by reoxygenation have been shown to generate bursts of free radicals that might exceed the antioxidant capacity of the lung and thereby cause tissue damage (15).
There was a weak correlation between lung HA concentration and water content in the present study, indicating that a large part of the tissue HA is part of the normal structure. The -1 d pups exposed to continuous hyperoxia at 6 d showed an approximate doubling of the HA concentration parallel with an approximate 20% increase in W/D ratio.
The body weight at death found in our study was lower than the birth weight during the first 2-3 postnatal days, and then increased in all groups; this is compatible with the weight changes normally seen in newborn infants. Compared with control pups, a smaller mean weight increase was observed in some groups of pups exposed to hyperoxia. Similar observations have been made by other investigators (30). Malnutrition may alter the inflammatory response (31) and therefore may also influence the HA synthesis.
HA is thought to be a major regulator of the water content in the extracellular matrix, where it also influences the mobility of cells and other cellular functions by environment modification and through direct action on specific cellular receptors (10). HA is synthesized in the plasma membrane of most mesenchymal cells, especially by activated fibroblasts. It is present in mammalian tissue matrix in large amounts during normal fetal development, and after injury. High concentrations are found during remodeling of tissues in the course of normal development as well as in diseased tissues (32). Many active substances in the inflammatory system (IL-1, platelet-derived growth factor, interferons, and prostaglandins) can stimulate fibroblasts to produce more HA (33). HA has a fairly rapid turnover in most tissues, with a t1/2 in the order of 1 d. This is partly due to lymphatic removal of HA from the tissues and subsequent degradation in lymph nodes and the liver, and partly to local endocytosis and degradation (32).
Increased concentrations of HA in bronchoalveolar lavage fluid and tissue biopsies have been observed in many inflammatory lung diseases (11,12,34–37). Increased lung HA concentrations have been found to covariate with disease severity, and with duration of mechanical ventilation up to 96 h in preterm monkeys with respiratory distress syndrome (38). Furthermore, in experimental alveolitis, accumulation of HA has been shown to parallel pulmonary edema (13,39).
The origin of the increased tissue HA concentrations in inflammation remains unclear. Both increased synthesis and reduced degradation have been reported. Juul et al. (40) found increased HA synthesis in mechanically ventilated preterm monkeys with respiratory distress syndrome. Teder and Heldin (41) found decreased HA degradation by alveolar macrophages without changes in lysosomal hyaluronidase activity in bleomycin-induced lung injury in rats. A small increase in hyaluronidase activity, less than the physiologic postnatal increase, was found 1 d, but not 3 d, after exposing adult rats to 100% oxygen for 60 h by Thet et al. (42).
Prolonged exposure of newborn animals to high concentrations of oxygen is known to cause pulmonary damage with a concomitant increase in the lung water content. Devaskar et al. (9) exposed newborn rats to a fractional concentration of inspired O2 of >85% for 12 d and found that they had a significantly higher lung water content than controls at the age of 4-8 d, with a maximum water content of 97.5% (comparable to a W/D ratio of 40) 6 d postpartum, considerably higher than the highest W/D ratio in the present study, i.e. 6.1 ± 0.2 (SD) 6 d postterm in -1 d rabbit pups. The reason for this difference is unclear, but it is probably not due to differences in species. Juul et al. (43) exposed term newborn rats to hyperoxia (>95% O2) for up to 10 d and found W/D ratios of the magnitude that we found in rabbit pups in the present study. In the study by Devaskar et al. (9), the rat lungs were probably more affected by hyperoxia than the lungs of the rabbit pups in the present study, as judged from the W/D ratio. Surprisingly, the mortality rates among the rats (9) and the rabbit pups (this study) were quite similar, namely 40 and 34%, respectively.
In the study by Juul et al. (43) on full-term rats from the 3rd to the 13th d of life, there was a steady decrease in the lung HA concentration in the control animals after birth, whereas in the present study this concentration showed a slight (nonsignificant) peak at term and 2 d postterm in both preterm and term groups. The rats exposed to hyperoxia in the study by Juul et al. (43) showed an increase in lung HA that reached a maximum at d 7 of exposure. All HA concentrations found in their study were considerably higher than those in the rabbits of the present study. However, in animals exposed to oxygen there was an increase in lung HA of the same proportion (approximately 100%) in both studies, as compared with control animals.
Histologic examination of lungs in untreated rabbit pups in our previous study (26) revealed that HA was abundant in the alveolar interstitial regions before term and then disappeared, whereas it remained in structural elements, such as perivascular and peribronchial tissue and in the pleura. Specific staining for HA in slices of rabbit lungs in the present study revealed that the excess HA in the hyperoxia-exposed lungs was located mainly in the perialveolar interstitium, and in bronchiolar and arteriolar walls. Juul et al. (43), working with neonatal rats exposed to hyperoxia, found an accumulation of HA in the alveolar wall, as in our study, but also in the perivascular cuffs of medium-sized arteries. With our method at 6 d postterm, the HA staining in the arteriolar walls in control pups averaged 2.2-2.6 on a 1-3 semiquantitative scale, whereas pups exposed to hyperoxia had average scores of 2.0-2.8. The high staining level in controls and the coarse scale may have concealed an increase in HA in this location. There may also be a difference due to differences in species and to gestational age.
The results of the present study indicate that oxygen exposure in the neonatal period may lead to an increase in the pulmonary HA concentration in parallel with an increase in the lung water content. The findings in the histologic examination indicate that the increased HA is located mainly in the perialveolar interstitium. The increase in the HA concentration in our experiments may be an effect of oxygen-induced stimulation of inflammatory mediators (33), or of oxygen free radicals (44). Reactions of this type may be of importance for the development of bronchopulmonary dysplasia (45).
In conclusion, after preterm or term birth, the lung HA concentration is unchanged in control rabbit pups, whereas the lung water content decreases. During exposure to hyperoxia, lung HA and lung water increase both in preterm and term rabbit pups, and are significantly higher at 4-6 d postterm than that in pups kept in room air. The increase in HA is most obvious around alveoli.
Abbreviations
- HA:
-
hyaluronan
- W/D:
-
wet/dry lung weight
- -2 d pups:
-
pups delivered 2 d before term
- -1 d pups:
-
pups delivered 1 d before term
References
Kitterman JA, Ballard PL, Clements JA, Mescher EJ, Tooley WH 1979 Tracheal fluid in fetal lambs: spontaneous decrease prior to birth. J Appl Physiol 47: 985–989
Bland RD 1983 Dynamics of pulmonary water before and after birth. Acta Paediatr Scand Suppl 305: 12–20
Bland RD, Bressack MA, McMillan DD 1979 Labor decreases the lung's water content of newborn rabbits. Am J Obstet Gynecol 135: 364–367
Krantz ME, Wennergren M, Bengtson LGW, Hjalmarson O, Karlsson K, Sellgren U 1986 Epidemiological analysis of the increased risk of disturbed neonatal respiratory adaptation after Caesarean section. Acta Paediatr Scand 75: 832–839
Bland RD 1983 Edema formation in the lungs and its relationship to neonatal respiratory distress. Acta Paediatr Scand Suppl 305: 92–99
Jackson JC, Truog WE, Standaert TA, Juul SE, Murphy JH, Chi EY, Mackenzie AP, Hodson WA 1991 Effect of high-frequency ventilation on the development of alveolar edema in premature monkeys at risk for hyaline membrane disease. Am Rev Respir Dis 143: 865–871
Saugstad OD 1996 Role of hypoxanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics 98: 103–107
Brigham KL 1990 Oxidant stress and adult respiratory distress syndrome. Eur Respir J 3( suppl 11): 482s–484s
Devaskar UP, Taylor W, Govindrajan R, Malicdem M, Heyman S, deMello DE 1994 Hyperoxia induces interstitial (type I) and increases type IV collagenase mRNA expression and increases type I and IV collagenolytic activity in newborn rat lung. Biol Neonate 66: 76–85
Fraser JRE, Laurent TC 1996 Hyaluronan. In: Comper WD (ed) Extracellular Matrix, Vol 2. Harwood Academic Publishers GmbH, Amsterdam, pp 141–199.
Sahu SC 1980 Hyaluronic acid. An indicator of pathological conditions of human lungs? Inflammation 4: 107–112
Modig J, Hällgren R 1989 Increased hyaluronan production in lung-a possible important factor in interstitial and alveolar edema during general anesthesia and in adult respiratory distress syndrome. Resuscitation 17: 223–231
Nettelbladt O, Tengblad A, Hällgren R 1989 Lung accumulation of hyaluronan parallels pulmonary edema in experimental alveolitis. Am J Physiol 257: L379–L384
Hernnäs J, Nettelbladt O, Bjermer L, Särnstrand B, Malmström A, Hällgren R 1992 Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J 5: 404–410
Kelly FJ 1993 Free radical disorders of preterm infants. Br Med Bull 49: 668–678
Frank L, Groseclose EE 1984 Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res 18: 240–244
Frank L, Sosenko IRS 1987 Prenatal development of lung antioxidant enzymes in four species. J Pediatr 110: 106–110
Frank L, Sosenko IRS 1991 Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. Pediatr Res 29: 292–296
Johnsson H, Sedin G, Jonzon A, Laurent T 1991 Water and hyaluronan content in the lungs in the perinatal period-an experimental study in rabbits. Pediatr Res 30: 656( abstr)
Johnsson H, Sedin G, Jonzon A, Laurent T 1992 Hyaluronan and water content in lungs from rabbit pups born preterm and subsequently kept in air or oxygen. Pediatr Res 32: 635( abstr)
Johnsson H, Sedin G, Jonzon A, Laurent T 1992 Hyaluronan and water content in lungs from preterm rabbit pups kept in air or oxygen. Biol Neonate 62: 180( abstr)
Johnsson H, Sedin G, Jonzon A, Laurent T 1994 Hyaluronan and water content in the lungs of term rabbit pups kept in air or oxygen. Pediatr Res 35: 280( abstr)
Sedin G, Gerdin B, Johnsson H, Jonzon A, Eriksson L, Hällgren R 1994 Hyaluronan and water content in the lung of preterm and term infants who died less than 24 hours after birth. Pediatr Res 36: 37A( abstr)
Hellström S, Tengblad A, Johansson C, Hedlund U, Axelsson E 1990 An improved technique for hyaluronan histochemistry using microwave irradiation. Histochem J 22: 677–682
Ripellino JA, Klinger MM, Margolis RU, Margolis RK 1985 The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. J Histochem Cytochem 33: 1060–1066
Allen SJ, Sedin G, Jonzon A, Wells AF, Laurent TC 1991 Lung hyaluronan during development: a quantitative and morphological study. Am J Physiol 260: H1449–H1454
Brandt R, Hedlöf E, Asman I, Bucht A, Tengblad A 1987 A convenient radiometric assay for hyaluronan. Acta Otolaryngol Suppl 442: 31–35
Presti D, Scott JE 1994 Hyaluronan-mediated protective effect against cell damage caused by enzymatically produced hydroxyl (OH˙) radicals is dependent on hyaluronan molecular mass. Cell Biochem Funct 12: 281–288
Cortivo R, Brun P, Cardarelli L, O'Reagan M, Radice M, Abatangelo G 1996 Antioxidant effects of hyaluronan and its α-methyl-prednisolone derivative in chondrocyte and cartilage cultures. Semin Arthritis Rheum 26: 492–501
Kelly FJ, Lubec G 1995 Hypoxic injury of immature guinea pig lung is mediated via hydroxyl radicals. Pediatr Res 38: 286–291
Lyoumi S, Tamion F, Petit J, Déchelotte P, Dauguet C, Scotté M, Hiron M, Liplingard A, Salier JP, Daveau M, Lebreton JP 1998 Induction and modulation of acute-phase response by protein malnutrition in rats: comparative effect of systemic and localized inflammation on interleukin-6 and acute-phase protein synthesis. J Nutr 128: 166–174
Laurent TC, Fraser JRE 1992 Hyaluronan. FASEB J 6: 2397–2404
Elias JA, Krol RC, Freundlich B, Sampson PM 1988 Regulation of human lung fibroblast glycosaminoglycan production by recombinant interferons, tumor necrosis factor, and lymphotoxin. J Clin Invest 81: 325–333
Nettelbladt O, Bergh J, Schenholm M, Tengblad A, Hällgren R 1989 Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis 139: 759–762
Hällgren R, Samuelsson T, Laurent TC, Modig J 1989 Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis 139: 682–687
Bjermer L, Engström-Laurent A, Thunell M, Hällgren R 1987 Hyaluronic acid in bronchoalveolar lavage fluid in patients with sarcoidosis: relationship to lavage mast cells. Thorax 42: 933–938
Hällgren R, Eklund A, Engström-Laurent A, Schmekel B 1985 Hyaluronate in bronchoalveolar lavage fluid: a new marker in sarcoidosis reflecting pulmonary disease. BMJ 113: 1–9
Juul S, Kinsella MG, Jackson JC, Truog WE, Standaert TA, Hodson A 1994 Changes in hyaluronan deposition during early respiratory distress syndrome in premature monkeys. Pediatr Res 35: 238–243
Anderson Bray B, Sampson PM, Osman M, Giandomenico A, Turino GM 1991 Early changes in lung tissue hyaluronan (hyaluronic acid) and hyaluronidase in Bleomycin-induced alveolitis in hamsters. Am Rev Respir Dis 143: 284–288
Juul SE, Kinsella MG, Wight TN, Hodson WA 1993 Alterations in nonhuman primate (M. nemestrina) lung proteoglycans during normal development and acute hyaline membrane disease. Am J Respir Cell Mol Biol 8: 299–310
Teder P, Heldin P 1997 Mechanism of impaired local hyalurona turnover in bleomycin-induced lung injury in rat. Am J Respir Cell Mol Biol 17: 376–85
Thet LA, Howell AC, Han G 1983 Changes in lung hyaluronidase activity associated with lung growth, injury and repair. Biochem Biophys Res Commun 117: 71–77
Juul SE, Krueger RC, Scofield L, Hershenson MB 1995 Hyperoxia alone causes changes in lung proteoglycans and hyaluronan in neonatal rat pups. Am J Respir Cell Mol Biol 13: 629–638
Tate RM, Vanbenthuysen KM, Shasby DM, McMurtry IF, Repine JE 1982 Oxygen-radical-mediated permeability edema and vasoconstriction is isolated perfused rabbit lungs. Am Rev Respir Dis 126: 802–806
Veness-Meehan KA, Rhodes DN, Stiles AD 1994 Temporal and spatial expression of biglycan in chronic oxygen-induced lung injury. Am J Respir Cell Mol Biol 11: 509–516
Acknowledgements
The authors thank Kajsa Lilja and Barbro Kjällström for expert technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by the Swedish Medical Research Council Grants 19X-4998 and 03X-4.
Rights and permissions
About this article
Cite this article
Johnsson, H., Eriksson, L., Jonzon, A. et al. Lung Hyaluronan and Water Content in Preterm and Term Rabbit Pups Exposed to Oxygen or Air. Pediatr Res 44, 716–722 (1998). https://doi.org/10.1203/00006450-199811000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199811000-00014
This article is cited by
-
CPAP-induced airway hyper-reactivity in mice is modulated by hyaluronan synthase-3
Pediatric Research (2022)