Abstract
Plasma creatinine levels are elevated in the first postnatal days, and the highest plasma creatinine values are observed in the most premature infants. These high plasma creatinine levels remain "elevated" beyond the period in which the high plasma creatinine levels can be explained by maternal transfer of creatinine. To better define the renal handling of creatinine by the immature kidney, creatinine and inulin clearances were simultaneously measured in two groups of neonatal and one group of adult anesthetized, ventilated rabbits. In the adult animals the ratio of the creatinine and inulin clearance was as expected more than one (1.21), indicating an overestimation of the true GFR due to tubular secretion of creatinine. The creatinine and inulin clearance ratio in the first group of newborn animals, who received an exogenous creatinine infusion to achieve plasma creatinine levels comparable to those in the adult animals (84.1 ± 1.0 µmol/L; 0.95 ± 0.01 mg/dL), was 0.84. When in the second group of neonatal animals the plasma creatinine level was artificially doubled to 155.0 ± 3.9 µmol/L (1.33 ± 0.17 mg/dL), no significant difference between both clearance values was found (ratio: 0.96). These data show that in the newborn rabbit creatinine is reabsorbed along the tubule, an observation that can best be explained by the back-leak of creatinine across leaky immature tubules.
Similar content being viewed by others
Main
Several years ago we published in this journal our observation that in the human neonate immediately after birth the plasma creatinine levels in preterm babies are significantly higher than in term infants (1). This difference persisted until approximately 3 wk after birth, much longer than could be accounted for by the maternal passage of creatinine, a small molecular mass substance (133 D, 0.3-nm radius) which is freely filterable at the placental barrier. In mammals urinary creatinine excretion is determined by glomerular filtration and to some extent by tubular secretion (2,3). Due to this tubular secretion of creatinine the creatinine clearance normally overestimates the inulin clearance, the gold standard of GFR. Two studies in neonates (piglets and humans) (4,5) seem to indicate that the creatinine clearance in these subjects underestimates GFR, suggesting that in the newborn there is tubular reabsorption of creatinine. The present studies were undertaken to evaluate the dynamics of the creatinine clearance in the newborn. The newborn rabbit is a good model for the study of renal function of the human premature infant, because in the rabbit nephrogenesis continues until approximately 10-14 d of postnatal life, thus reflecting the renal immaturity present in the premature human infant (6–8)
METHODS
Two comparable groups of 5-10-d-old newborn (groups 1 and 2) and a group of adult (group 3) New Zealand White rabbits were studied.
Preparation. In the newborn animals anesthesia was initiated with an intraperitoneal dose of 25 mg/kg of a 0.5% sodium pentobarbital solution; the adult animals received an i.v. dose of 30 mg/kg. Additional, small i.v. doses were given when indicated. All animals were mechanically ventilated through a tracheotomy with a rodent ventilator (Harvard 683, Millis, MA). The respiratory rate was kept constant at 40 breaths/min, and tidal volume was adjusted for age and weight. With a heating table and an infrared light, the body temperature of the animals was kept constant between 38.0 and 38.5°C, monitored with an intraesophageal thermometer (Hewlett Packard temperature recorder 78231C, Palo Alto, CA). The femoral artery and vein were catheterized with appropriately sized tubing for blood sampling, fluid administration, as well as measurements of mean arterial blood pressure and heart rate. The bladder was catheterized for urine collection. Statham transducers recorded arterial and ventilatory pressures (model 7B polygraph, Grass Instruments, Quincy, MA).
After surgery the animals received an i.v. priming dose of inulin (100 mg/kg), followed by a continuous infusion (10 mL kg-1 h-1) of a modified rabbit Ringer's solution containing per liter 50 g of mannitol, 3 g of inulin (groups 1 and 2), or 20 g of inulin (group 3), 150 mmol of sodium, 105 mmol of chloride, 5 mmol of potassium, and 50 mmol of sodium bicarbonate. The preparation of the animals took approximately 1 h. All rabbits were studied under normoxemic conditions. None of the rabbits was exposed to drugs or chemicals that are known to interfere with tubular creatinine transport. After the surgical procedures, a 90-min equilibration period was allowed, followed by four 30-min (groups 1 and 2) or 20-min (group 3) urine collections. Blood (0.4 mL) was withdrawn at the midpoint of each urine collection. The red blood cells were reconstituted in 0.4 mL of human albumin and reinfused.
Animals. Group 1 (n = 9): the animals in this group of neonatal animals had a weight of 116.8 ± 7.8 g (X ± SEM) and a basic plasma creatinine level of 30-50 µmol/L (0.34-0.45 mg/dL), which was artificially raised to 74.4 ± 7.8 µmol/L (0.85 ± 0.02 mg/dL) by the addition of creatinine (Sigma Chemical Co., Buchs, Switzerland) to the constant infusion (vide supra), both as a loading dose (330 µmol/L) and as a sustaining dose (0.33 µmol/L). Group 2 (n = 11): these newborn animals had the same weight (116.0 ± 18.5 g) and the same basic plasma creatinine levels (30-50 µmol/L, 0.34-0.45 mg/dL) as the animals in group 1, but their plasma creatinine level was raised to 155.0 ± 3.9 µmol/L (1.75 ± 0.04 mg/dL) with the doubling of the exogenous administration of creatinine (loading dose, 660 µmol/L; sustaining dose, 0.66 µmol/L). Group 3 (n = 8): the adult animals in this group weighed 2100 ± 250 g; their baseline plasma creatinine level was 84.1 ± 1.0 µmol/L (0.95 ± 0.01 mg/dL) (see Table 1). In these adult animals basic endogenous creatinine and exogenous inulin clearance were determined; no exogenous creatinine was added.
Laboratory techniques. Creatinine and inulin in blood and urine were measured with the creatinine kinetics (BioMérieux, Lyon, France) and the anthrone methods, respectively (9,10).
Statistics. All data are expressed as mean ± SEM. The significance of the changes were tested with the paired t test. A p < 0.05 was considered significant. The clearance values were corrected for kilograms of body weight (11).
RESULTS
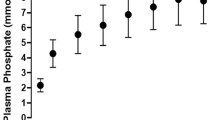
The results are summarized in Table 1. The addition of a relatively small amount of exogenous creatinine raised the plasma creatinine level of the animals of group 1 from a value of 30-50 µmol/L (0.34-0.45 mg/dL) to 74.4 ± 7.8 µmol/L (0.85 ± 0.02 mg/dL) (Table 1). Doubling the amount of creatinine in the infusion of the animals of group 2 more than doubled (×2.1) the plasma creatinine level with only a relatively small rise in the creatinine clearance (+17.7%). This latter value (1.33 ± 0.17 mL min-1 kg-1) was still much lower (a factor of 2.6) than the creatinine clearance observed in the adult animals of group 3 (Table 1). The most interesting findings of these experiments, however, were obtained when the creatinine and inulin clearances in each group of animals were compared. In the adult animals of group 3 the creatinine clearance was 21% greater than the inulin clearance, the universally accepted best measurement of GFR. In these animals the creatinine clearance thus overestimated true GFR. In contrast, the creatinine clearance underestimated GFR in the newborn animals of group 1 (-16%). This is graphically depicted in Figure 1. In the neonatal animals of group 2 the creatinine and inulin clearances were virtually identical (-4%). The mean arterial pressure and the heart rate were stable in all experiments.
DISCUSSION
GFR in the human neonate and in newborn animals such as the rabbit is very low, even corrected for kidney mass, body surface, and total body water (12). This major limitation of neonatal renal function poses great practical difficulties in measuring GFR at this very young age. Inulin is the gold standard for the measurement of GFR at any age. For matters of convenience, however, the endogenous creatinine clearance is generally used in clinical practice and clinical research (13–15). One of the basic requirements for the validity of the creatinine clearance is that plasma creatinine levels remain constant during a finite period of time. Under steady state conditions the plasma creatinine level is dependent on total body muscle mass, which in older children and healthy adults does not change appreciably. This is not true in the rapidly growing neonates in whom the stable periods of creatinine production are rather short. This is even more pronounced in premature babies whose plasma creatinine levels are much higher than can be expected for their body size (1). Despite these basic limitations, even in the neonate the short time stable plasma creatinine level and the fact that creatinine is completely filtered at the glomerular filtration barrier, come close to fulfil the criteria for the two main conditions for an ideal marker of GFR. The third condition, that creatinine is not handled by the tubule, is less well controlled at all ages. In the adult, the endogenous creatinine clearance overestimates GFR by a factor of 20-40%, although this figure is rather variable (2,3). The overestimation is due to tubular secretion of creatinine. Both Smith (2) and Pitts (3) emphasized, and this is clearly reiterated in a modern textbook of physiology (16), that when a certain substance is only filtered and not reabsorbed nor secreted along the renal tubule, the clearance of that substance equals the clearance of inulin. To quote Guyton and Hall (16): "If the clearance rate of the substance is greater than the inulin clearance, the substance must be secreted by the nephron tubules and if the clearance rate is less than the inulin clearance, the substance must have been reabsorbed."
In the present experiments, the normally occurring overestimation of the creatinine clearance was indeed seen in the adult animals of group 3 (Table 1). In the neonatal animals, however, the opposite phenomenon was observed. At a level of plasma creatinine, which was artificially raised to be in the same range as that of the adult animals, a definite underestimation of the GFR was found (group 1). The only valid explanation for this finding is that in the neonate creatinine is reabsorbed along the tubule. When the already high plasma creatinine level in the newborn animals of group 1 was even raised further (group 2), the creatinine clearance increased relatively little, whereas at this point there was virtually no difference between the clearances of creatinine and inulin. Because plasma creatinine doubled and the urine flow remained unchanged (the amount of exogenous creatinine was doubled, not the rate of the infusion), the urinary excretion of creatinine must have almost doubled as well. In other words, the tubular reabsorption of creatinine had previously already almost reached its maximal capacity, and after the doubling of the plasma creatinine level the remainder of the filtered load of creatinine was excreted unchanged in the final urine sample.
Tubular reabsorption of creatinine is a very unusual physiologic phenomenon. There are, however, previous data which support our experimental findings. In studies on fetal piglets, undertaken to evaluate the existence of glomerulotubular balance in the developing kidney, Alt et al. (4) state that net tubular reabsorption of creatinine was found. In premature and term infants Coulthard et al. (5) also observed that creatinine clearances were lower than inulin clearances; these authors attributed their findings to the techniques used for measuring creatinine. In dehydrated human adult volunteers with a low urinary flow, tubular reabsorption of creatinine was also observed (17).
The present study is the first to unequivocally show, in an animal experimental setting, that immediately after birth the creatinine clearance underestimates "true" GFR. These data, which were obtained by the simultaneous measurement of creatinine and inulin clearances in newborn rabbits, are physiologically applicable to premature human infants. It may well be that tubular reabsorption of creatinine is a physiologic phenomenon in the "immature" developing kidney. We propose that this is due to a slow urinary flow along the tubule at this age and in particular to increased back-leak of creatinine along the immature tubular structures.
It is refreshing to find that in this day and age of ever more sophisticated biologic study methods, an astute clinical observation can still be "answered" by time-proven basic renal physiologic studies. For obvious reasons these studies had to be performed in an animal model and could not be verified in the human neonate. The reasons why and precisely how creatinine is reabsorbed along the "immature" tubules need further study.
References
Bueva A A, Guignard 1994 Renal function in preterm neonates. Pediatr Res 36: 572–577
Smith HW 1951 The Kidney, Structure and Function in Health and Disease. Oxford University Press, New York, Chap. 3, pp 39-63; Chap. 7, 182–194.
Pitts RF 1974 Physiology of the Kidney and Body Fluids. 3rd Ed. Year Book, Chicago, Chap. 4-8, 49–157.
Alt JM, Colenbrander B, Forsling ML, MacDonald AA 1984 Perinatal development of tubular function in the pig. Q J Exp Physiol 69: 693–702
Coulthard MG, Hey EN, Ruddock V 1985 Creatinine and urea clearances compared to inulin clearance in preterm and mature babies. Early Hum Dev 11: 11–19
Jaton T, Thonney M, Gouyon JB, Guignard, J-P 1992 Renal effects of dopamine and dopexamine in the newborn anesthetized rabbit. Life Sci 50: 195–202
Semama DS, Thonney M, Guignard, J-P 1993 Role of endogenous endothelin in renal hemodynamics of newborn rabbits. Pediatr Nephrol 7: 886–890
Ballèvre L, Thonney M, Guignard, J-P 1966 Nitric oxide modulates filtration and renal blood flow of the newborn rabbit. Biol Neonate 69: 389–398
Bartels H, Böhmer M, Heierli C 1972 Serum Kreatininebestimmung ohne Enteiweissen. Clin Chim Acta 37: 193–197
Wright HK, Gann DS 1966 An automatic anthrone method for the determination of inulin in plasma and urine. J Lab Clin Med 67: 689–693
Coulthard MG, Hey EN 1984 Weight as the best standard for glomerular filtration in the newborn. Arch Dis Child 59: 373–375
McCrory WW 1972 Developmental Nephrology. Harvard University Press, Cambridge MA, Chap. 3, 79–122.
Mattar G, Barnett HL, McNamara H, Lauson HD 1952 Measurement of glomerular filtration rate in children with kidney disease. J Clin Invest 31: 938–944
Alinei P, Guignard J-P 1987 Assessment of glomerular filtration rate in infants. Comparison of three methods used in clinical pediatrics. Helv Paediatr Acta 42: 253–262
Schwartz GJ, Brion LP, Spitzer A 1987 The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children and adolescents. Pediatr Clin North Am 34: 571–590
Guyton AG, Hall JE 1996 Textbook of Medical Physiology. WB Saunders, Philadelphia, 348
Sjöström PA, Odlind BG, Wolgast M 1988 Extensive tubular secretion and reabsorption of creatinine in humans. Scand J Urol Nephrol 22: 129–131
Author information
Authors and Affiliations
Additional information
Supported by the National Swiss Science Foundation 3200-036574.92 and 3200-052463.97.
Rights and permissions
About this article
Cite this article
Matos, P., Duarte-Silva, M., Drukker, A. et al. Creatinine Reabsorption by the Newborn Rabbit Kidney. Pediatr Res 44, 639–641 (1998). https://doi.org/10.1203/00006450-199811000-00004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199811000-00004
This article is cited by
-
Effect of Kidney Function on Drug Kinetics and Dosing in Neonates, Infants, and Children
Clinical Pharmacokinetics (2015)
-
The interplay between drugs and the kidney in premature neonates
Pediatric Nephrology (2014)
-
Renal creatinine handling in very old patients with chronic renal disease
International Urology and Nephrology (2011)
-
Creatinine reabsorption by the aged kidney
International Urology and Nephrology (2009)