Abstract
Patients with a microdeletion on chromosome 22q11 demonstrate the clinical picture of the velocardiofacial syndrome. We report on three members of the same family with this microdeletion and velocardiofacial syndrome, all having an increase in platelet size and a mild decrease in platelet number. Their platelet function, however, tested by aggregation and by adherence to collagen in a whole blood perfusion system, was normal. We retrospectively studied the files of 35 other patients with 22q11 deletion and also found that their platelets had an increased size compared with cardiac controls. Moreover, their platelet size correlated negatively with platelet number. Knowing that patients with 22q11 deletion are obligate carriers for a heterozygous glycoprotein Ibβ deletion, these patients can be considered to be heterozygous Bernard-Soulier patients. In addition, a significant increase in platelet size may be a positive predictor for the clinical diagnosis of the velocardiofacial syndrome.
Similar content being viewed by others
Main
The clinical picture of patients with 22q11 is very broad, from the more severe DiGeorge sequence (thymic hypoplasia, conotruncal cardiac defects, and facial abnormalities) it ranges to the milder VCFS (velopharyngeal insufficiency, conotruncal heart disease, and learning disabilities) (1). To date, only two reports describe platelet abnormalities associated with this disorder. Levy et al. (2) report on two female carriers of del 22q11, accompanied by thrombocytopenia. The first of these patients demonstrated all aspects of idiopathic thrombocytopenic purpura, whereas the cause of thrombocytopenia in the second patient was not documented. Budarf et al. (3) described one boy with del 22q11 and BSS. BSS is a recessive disorder commonly caused by a homozygous GP Ibα gene dysfunction, resulting in no normal GP Ib/IX/V complex on the platelet membrane, and characterized by thrombocytopenia, enlarged platelets and decreased or absent GP Ib/IX/V on the platelet membrane (4).
The GP Ib/IX/V complex is the platelet receptor for von Willebrand factor and initiates platelet adhesion to injured subendothelium (5). GP Ib is a disulfide-linked heterodimer of GP Ibα and GP Ibβ and, together with GP IX and GP V, it forms a noncovalent but stable membrane complex.
The patient described by Budarf et al. (3) was further investigated by Ludlow et al. (6), who found that this BSS patient was a compound heterozygote with the deletion of one allele of the GP Ibβ gene, that maps on the deleted 22q11.2 region, and a mutated GATA binding site in the promotor of the remaining GP Ibβ allele.
We present here three patients from one family with a del 22q11, mild thrombocytopenia, and increased platelet size. In addition, the retrospective analysis of 35 patients with del 22q11 and known peripheral blood counts revealed an increase in platelet size in these patients and a significant negative correlation between their platelet numbers and platelet size.
METHODS
Patient populations. The index patient (patient 1) in the present family is a boy, with tetralogy of Fallot, a velopharyngeal insufficiency, facial dysmorphism, and moderate mental retardation. The high resolution karyotype of this patient was normal after G-banding in white blood cells and fibroblasts. A submicroscopic deletion in 22q11 was demonstrated by FISH, using probe D0832. His younger sister (patient 2) is moderately mentally retarded and has facial dysmorphism and velopharyngeal insufficiency, for which she underwent a pharyngoplasty at the age of 6 y without bleeding problems. She also has del 22q11. The father of these two children (patient 3) functions on a borderline mental level. He has a hypernasal speech and typical facial features of VCFS. He suffers from psoriasis, and from the age of 7 y, he has total alopecia of unknown cause. A del 22q11 was demonstrated. Another sister of the propositus and the mother are physically and mentally normal and have no del 22q11.
We also studied a group of 35 other patients with features of VCFS and a del 22q11, determined by FISH using probe D0832, of whom 26 had a characteristic conotruncal heart defect. We retrospectively examined the medical files of these patients for whom peripheral blood counts and MPVs were available.
As a control group, we included 49 patients without del 22q11 but with congenital conotruncal heart defects similar to those found in the VCFS. Also for these patients, peripheral blood counts and MPVs were available.
Fluorescence in situ hybridization. FISH to demonstrate a del 22q11 was done using probe D0832-D22S931 as described previously (7).
Platelet analysis. Platelet counts and MPV were determined automatically on a Coulter counter. In the family presented, the platelet count was also determined manually with the aid of microscopic cell counting; the platelet size was determined on a blood smear microscopic slide, via a microscopic image analysis program (Quantimed, Leica), and automated surface analysis of individual platelets. Platelets antibodies were studied using a solid phase red cell adherence assay (Capture PR, Immucor Inc., Norcross, GA).
Platelet aggregation was studied as described previously (8); blood anticoagulated with 3.13% citrate was centrifuged at 1000 rpm to produce platelet-rich plasma. Aggregation in this platelet-rich plasma was induced by ADP (2.5 and 5 µM), collagen (1 and 2 µg/mL), ristocetin (0.5-1.2 mg/mL), arachidonic acid (1 mM), and U46619 (thromboxane analog), in a dual channel Chronolog Aggregometer. Maximal amplitude, maximal velocity, and lag phase were determined. Primary hemostasis in vivo was studied with the Ivy bleeding time.
Platelet adhesion to collagen. Platelet adhesion studies to collagen were performed as described previously (9) using a parallel plate perfusion chamber in vitro. Briefly, platelet deposition onto calf skin collagen-coated coverslips was determined by recirculating patient blood anticoagulated with low molecular weight heparin (0.2 U/mL) through flow chambers with the adequate chamber height (0.4-1 mm) at a fixed flow rate of 45 mL/min. Perfusions were carried out for 2 min; thermanox coverslips had been coated overnight with calf skin collagen (Sigma Chemical Co., St. Louis, MO; 1 mg/mL in 50 mmol/L acetic acid). Coverslips were removed from the perfusion chamber, rinsed with HEPES buffer (pH 7.5), and fixed with 1% glutaraldehyde in PBS before staining with May-Grünwald-Giemsa. Quantification of platelet adhesion was done by image analysis of the surface covered using en face light microscopy (Dialux 20 EB, E, Leitz GmbH, Wetzlar, FRG) at low magnification (×40) and the "TCL-image" image processing software (Multihouse TSI, Amsterdam, The Netherlands). The disappearance of single platelets during the perfusion was calculated using the formula: single platelet disappearance = (final platelet count in EDTA - final platelet count in formol)/initial platelet count in EDTA. The platelet count of the blood samples was determined before and after perfusion with a Cell-Dyn 1300 (Abbott, Diagnostics Division).
The relation between the degree of platelet adhesion and the blood platelet count was established via linear regression of the platelet surface coverage (%) versus platelet numbers for perfusions carried out with blood samples obtained from 40 patients scheduled for minor surgery.
Statistical analysis. Linear regression was performed using the GRAFIT graphical program for Windows, version 3. Statistical analysis of the significance of correlation was analyzed via the calculation of p values, using the INSTAT 2 statistical software program for Windows. To calculate median values and confidence intervals, INSTAT 2 was equally used. To compared the platelet volume distribution in patients with and without del 22q11 and with and without cardiac problems, the Mann-Whitney U test was used.
RESULTS
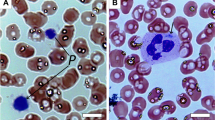
The index patient had normal to mildly decreased platelet numbers (Table 1) and underwent cardiac surgery without bleeding problems. The younger sister carrying a del 22q11 (patient 2) equally had mildly low platelet numbers (Table 1). On several occasions, giant platelets were observed during direct blood smear examination. No platelet antibodies were found in patient 2. She underwent pharyngoplastic surgery without prolonged bleeding and her Ivy bleeding time was normal. The father (patient 3) has been followed for several years with apparently isolated, asymptomatic thrombocytopenia (Table 1). Pseudothrombocytopenia was excluded by using different anticoagulants. Light microscopic examination of bone marrow yielded a normal megakaryocyte number and morphology. Additional immunologic investigations, including platelet antibodies, were negative. The MPV for all three patients and determined on several occasions was elevated, and giant platelets were observed on direct microscopic examination. Manually determined platelet counts and platelet volumes compared reasonably well with the electronically determined values. The mother and sister who had no del 22q11 had normal platelet counts and morphology.
Platelet aggregation studies with ADP, collagen, ristocetin, arachidonic acid, and U46619 were not significantly different between the patients with the deletion, compared with their normal family members. Therefore, no platelet dysfunction could be detected via aggregometry. Platelet adhesion studies in the whole blood perfusion chambers were carried out for all members of this family, and the results are summarized in Table 2. The single platelet disappearance after perfusions of all samples was less then 30%. In view of the observation that the degree of platelet adhesion in a reference population of 40 patients was proportional to the platelet count (slope = 0.09; r = 0.42; p < 0.01), the low platelet adhesion observed for patient 3 relates to his low platelet number, rather than to an intrinsic deficiency of platelet adherence per se.
To further investigate the relation between platelet numbers and platelet morphology, a retrospective analysis was performed on 35 additional patients (20 boys and 15 girls; median age = 3 y, 5 mo, range = 7 d to 18 y), with proven del 22q11 and features of VCFS. In this group, 9 patients had no cardiac defects and 26 suffered from conotruncal heart defects (tetralogy of Fallot, n = 16; ventricular septal defect, n = 4; common arterial trunk, n = 4; interrupted aortic arch, n = 1; and ventricular septal defect combined with open ductus Botalli, n = 1).
Figure 1 shows the distribution of the MPV in the control group (49 cardiac patients without del 22q11) and in the patient group with del 22q11 (the 35 patients retrospectively studied and the presented 3 patients). The median MPV (11.05 fL) for the 38 patients with del 22q11 is significantly higher than for the control population of 49 patients (8.4 fL) (p < 0.000001). Both populations appear to have a Gaussian distribution pattern with 99% confidence limits ranging from 7.8 to 8.7 fL for the control group and from 10.3 to 11.8 fL for the group with del 22q11.
There is a strong negative correlation between the MPV and platelet count in the patients with del 22q11 (correlation coefficient - 0.583; slope = -0.016; p < 0.0001), whereas there is no correlation between these variables in the control group as demonstrated in Figure 2.
In addition, in the patients with del 22q11, the MPV of the 27 patients with congenital heart disease (median MPV 10.8 fL) does not differ significantly from the subpopulation of 11 patients without cardiac abnormalities (median MPV 11.5 fL).
DISCUSSION
Patients suffering from the DiGeorge/VCFS do not manifest an increased bleeding tendency. Especially at the time of the cardiac catheterization, cardiac surgery, or pharyngoplasty, these patients do not bleed extensively. The increase in platelet size and mild decrease in platelet number of the affected members in the family presented here, however, suggested abnormalities of the platelet membrane, associated with their del 22q11. The significance of this abnormality in platelet size and count was substantiated by the retrospective analysis of the hemograms of 35 other patients with del 22q11. Although, in this retrospective analysis, platelet counting and platelet size determinations were performed only via electronic counting, we believe that the calculated numbers are representative, in view of the good correlation between the electronically and manually determined values in the presently described family. Nonetheless, the risk is real that the largest platelets are not included in the analysis. However, if this were the case, the presently reported differences between a reference population of 49 patients with cardiac defects, but without 22q11 deletion, and the actual study group, would be even more pronounced (see below).
In many hematologic disorders, large platelets can be found. However, in the members of the presenting family, immunothrombocytopenia, May-Hegglin anomaly, and gray platelet syndrome could be excluded due to absence of anti-platelet antibodies or white blood cell anomalies and a normal platelet function, respectively. Also BSS (homozygote deficiency or compound heterozygote deficiency of the GP Ib/IX/V complex) could be excluded because of the absence of a bleeding problem and normal response of the platelets to ristocetin-induced aggregations.
Recent studies mapped GP Ibβ to chromosome band 22q11.2, by using a somatic cell hybrid mapping panel (10) and by applying FISH (11). Furthermore, subregional localization of the GP Ibβ gene demonstrates that this gene is located in the vicinity of probe D0832, which is within the region deleted in the vast majority of patients with a del 22q11 (12). Thus, the vast majority of patients with a del 22q11 are obligate heterozygous carriers for the GP Ibβ deletion, and therefore heterozygotes for BSS. Heterozygote carriers for BSS caused by a GP Ibα mutation also do not have a bleeding diathesis, but show giant platelets (13).
The decreased platelet number and increased platelet size in these 22q11-deleted patients could theoretically be attributed to increased platelet turnover due to mechanical disruption by the cardiac anomaly. However, this can be excluded because of two findings; the platelet size is significantly higher in the 22q11 patients than in the cardiac control group and furthermore in the 22q11-deleted patients the MPV of the patients with cardiac anomalies does not differ from the MPV of the patients without cardiac anomalies. A probable explanation is that platelet genesis depends on the GP Ib/V/IX complex-membrane density and that a deficiency of this complex leads to bigger and thus less platelets. Indeed, Nurden et al. (14) summarized evidence that the lack of GP Ib/V/IX complexes in the BSS results in thrombocytopenia and giant platelets due to the importance of GP Ib-IX for normal megakaryocyte maturation and demarcation membrane system production. On the other hand, decreased GP Ib/V/IX complex density on the platelet membrane of patients with BSS seems to correlate also with decreased platelet life span (13).
In conclusion, it follows that patients with del 22q11 have giant platelets most likely due to the obligate carriership for a GP Ibβ deficiency, but that this abnormality will not per se induce a bleeding tendency. The degree of thrombocytopenia will be the most important risk factor predicting bleeding. The elevated MPV suggests a high predictive value for the use of the MPV as a positive marker in the clinical diagnosis of the VCFS, a finding that will, however, require further investigation. The availability in our center of a large panel of patients with del 22q11 will enable such further studies, investigating variations in membrane density of GP Ib, V, and IX membrane proteins on the giant platelets found in these patients and will enable us to establish the relation between platelet morphology and GP Ib/V/IX membrane density.
Abbreviations
- BSS:
-
Bernard-Soulier syndrome
- del 22q11:
-
microdeletion on chromosome 22q11
- FISH:
-
fluorescence in situ hybridization
- GP:
-
glycoprotein
- MPV:
-
mean platelet volume
- VCFS:
-
velocardiofacial syndrome
REFERENCES
Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Schuffenhauer S, Oechsler H, Belohradsky B, Prieur M, Aurias A, Raymond FL, Clayton-Smith J, Hatchwell E, McKeown C, Beemer FA, Dallapiccola B, Novelli G, Hurst JA, Ignatius J, Green AJ, Winter RM, Brueton L, Brondum-Nielsen K 1997 Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet 34: 798–804
Lévy A, Michel G, Lemerrer M, Philip N 1997 Idiopathic thrombocytopenic purpura in two mothers of children with DiGeorge sequence: a new component manifestation of Catch 22. Am J Med Genet 69: 356–359
Budarf ML, Konkie BA, Ludlow LB, Michaud D, Li M, Yamashiro DJ, McDonald-McGinn D, Zackai E, Driscoll DA 1995 Identification of a patient with Bernard-Soulier syndrome and a deletion in the Digeorge/velo-cardio-facial chromosomal region in 22q 11.2. Hum Mol Gen 4: 763–766
De la Salle C, Lanza F, Cazenave JP 1995 Biochemical and molecular basis of Bernard-Soulier syndrome: a review. Nouv Rev Fr Hematol 37: 215–222
Sakariassen KS, Nievelstein PF, Colber BS, Sixma JJ 1996 The role of platelet membrane glycoproteins Ib and IIb/IIIa in platelet adherence to human artery subendothelium. Br J Haematol 63: 681–691
Ludlow LB, Schick BP, Budarf ML, Driscoll DA, Zackai EH, Cohen A, Konkle BA 1996 Identification of a mutation in a GATA binding site of the platelet glycoprotein Ibβ promotor resulting in the Bernard-Soulier syndrome. J Biol Chem 271: 22076–22080
Wadey R, Daw S, Wickremasinghe A, Roberts C, Wilson D, Goodship J, Burn J, Halford S, Scambler PJ 1993 Isolation of a new marker and conserved sequences close to the DiGeorge syndrome marker HP500 (D22S134). J Med Genet 30: 818–821
Deckmyn H, Stanssens P, Hoet B, Declerck PJ, Lauwereys M, Gansemans Y, Tornai I, Vermylen J 1994 An echistatin-like Arg-Gly-Asp (RGD)-containing sequence in the heavy chain CDR3 of a murine monoclonal antibody that inhibits human platelet glycoprotein IIb/IIIa function. Br J Haematol 87: 562–571
Yamamoto H, Vreys I, Stassen JM, Yoshimoto R, Vermylen J, Hoylaerts MF 1998 Injury induced arterial and venous thrombosis in the hamster are both inhibited by antagonism of vWF. Thromb Haemostasis 79: 202–210
Kelly MD, Essex DW, Shapiro SS, Meloni FJ, Druck T, Huebner K, Konkle BA 1994 Complementary DNA cloning of the alternatively expressed endothelial cell glycoprotein Ib β and localization of the GPIb β gene to chromosome 22. J Clin Invest 93: 2417–2424
Yagi M, Edelhoff S, Disteche CM, Roth GJ 1994 Structural characterization and chromosomal location of the gene encoding human platelet glycoprotein Ibβ. J Biol Chem 269: 17424–17427
Carlson C, Sirotkin H, Pandita R, Goldberg R, McKie J, Wadey R, Patanjali SR, Weissman SM, Anyane-Yeboa K, Warburton D, Scambler P, Shprintzen R, Kucher-lapati R, Murrow BE 1997 Molecular definition of 22q11 deletions in 151 velo-cardio-facial syndrome patients. Am J Hum Genet 61: 620–629
Tomer A, Scharf RE, McMillan R, Ruggeri ZM, Harker LA 1994 Bernard-Soulier syndrome: quantitative characterization of megakaryocytes and platelets by flow cytometric and platelet kinetic measurements. Eur J Haematol 52: 193–200
Nurden P, Poujol C, Nurden AT 1997 The evolution of megakaryocytes to platelets. Bailliere's Clin Haematol 10: 1–27
Acknowledgements
The authors thank Dr. P. Scambler, Institute of Child Health London, for his kind gift of probe D0832-D22S931.
Author information
Authors and Affiliations
Additional information
Supported by Grant G.0306.98 of the Belgian Fund for Scientific Research (FWO). J.V. is holder of the Dr. J. Choay Chair in Hemostasis Research.
Rights and permissions
About this article
Cite this article
Van Geet, C., Devriendt, K., Eyskens, B. et al. Velocardiofacial Syndrome Patients with a Heterozygous Chromosome 22q11 Deletion Have Giant Platelets. Pediatr Res 44, 607–611 (1998). https://doi.org/10.1203/00006450-199810000-00023
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199810000-00023
This article is cited by
-
Inherited Macrothrombocytopenia: Correlating Morphology, Epidemiology, Molecular Pathology and Clinical Features
Indian Journal of Hematology and Blood Transfusion (2018)
-
Effect of 22q11.2 deletion on bleeding and transfusion utilization in children with congenital heart disease undergoing cardiac surgery
Pediatric Research (2016)
-
Erblich bedingte Thrombozytopenien
Monatsschrift Kinderheilkunde (2006)
-
Hematologic Abnormalities in Severe Neonatal Necrotizing Enterocolitis: 25 Years Later
Journal of Perinatology (2003)
-
Genetic Abnormalities of Bernard-Soulier Syndrome
International Journal of Hematology (2002)