Abstract
Intrauterine growth restriction (IUGR) is characterized by a reduction in fetal plasma concentrations of a number of essential amino acids. Whether this is caused by impaired placental transport is unknown. We studied transport of leucine and lysine in syncytiotrophoblast microvillous (MVM) and basal membrane (BM) vesicles isolated from uncomplicated (control) and IUGR pregnancies. In addition, we investigated the possibility that leucine uptake is stimulated by an outwardly directed glycine gradient. Uptake of 3H-L-lysine (0.1 µM) and 3H-L-leucine (0.25 µM) was studied at 37°C using rapid filtration techniques. In IUGR, mediated uptake of lysine was reduced by 44% (p < 0.05) in BM and uptake of leucine was lower in both MVM (-46%, p < 0.05) and BM (-38%, p < 0.05) compared with control vesicles. Intravesicular glycine (2 mM) increased the uptake of leucine by 98% in MVM (p < 0.05). These data suggest that the activity of placental transporters for cationic and neutral amino acids is reduced in IUGR. We speculate that a reduced glycine gradient in the placenta in IUGR, due to reduction in system A activity, will impair leucine transport to the fetus, providing an additional mechanism for reduced placental transport of leucine in IUGR.
Similar content being viewed by others
Main
IUGR remains a significant obstetric and pediatric problem. IUGR is associated with increased perinatal morbidity (1), higher incidence of neurodevelopmental impairment (2), and possibly increased risk of cardiovascular disease and diabetes in adult age (3,4). Fetal growth is intimately related to placental growth and transplacental nutrient supply (5). To better understand the pathophysiology of IUGR, detailed information concerning mechanisms for transplacental transport of nutrients in normal and reduced fetal growth is needed. Furthermore, in designing new intervention strategies, including supplementation with metabolic substrates, any alteration in placental transporter activity in IUGR must be known.
In many cases of IUGR, "placental insufficiency" is assumed to be the immediate cause of the restricted intrauterine growth. However, it is not well established which specific functions of the placenta might be impaired in this pregnancy complication. The IUGR fetus is often hypoxic, hypoglycemic, and sometimes acidotic in utero (6,7). In addition, fetal plasma concentrations of a number of amino acids are reduced (8). These findings suggest that a reduced placental transport capacity is an important cause for the altered growth pattern in the IUGR fetus. However, we were unable to find any reductions in placental glucose transporter expression or activity in IUGR (9), suggesting that fetal hypoglycemia in IUGR is caused by other factors. In contrast, the activity of system A amino acid transporters in syncytiotrophoblast MVMs is reduced in IUGR (10,11). System A is a sodium-dependent transporter mediating the uptake of mainly nonessential amino acids such as glycine and alanine. However, a series of studies employing cordocentesis demonstrate that it is mostly the plasma concentrations of essential amino acids, such as lysine and leucine, that is reduced in the IUGR fetus (8,12,13). Essential amino acids are transported across the human placenta mediated by sodium-independent transport systems rather than by system A (14). The main objective of the present study was therefore to study the transport of lysine and leucine in syncytiotrophoblast plasma membranes isolated from IUGR placentas.
The intracellular concentrations of leucine in human syncytiotrophoblast are approximately six times higher than in maternal plasma (15). This uphill transport is difficult to explain considering that the transport of this neutral amino acid is sodium-independent. Glycine is a nonessential amino acid that is highly concentrated in the placenta by the Na+ -dependent system A transporter, but transfer of glycine to the fetus appears to be limited (16). In addition to system A, glycine is also accepted by system L, a transporter with broad specificity and affinity for most neutral amino acids such as leucine and phenylalanine. Glycine could be released from the cell mediated by system L and thereby stimulate the uptake of other neutral amino acids via this transporter. Interactions between amino acids concentrated into the cell by a highly energized transport system and sodium-independent transport systems is well known in amino acid transport physiology (17). However, these interactions are less well characterized for the human placenta. We hypothesized that the steep outwardly directed glycine gradient in human syncytiotrophoblast might drive uphill leucine transport by trans-stimulation of the system L. If so, a decreased glycine gradient in IUGR due to the reduced system A activity would constitute a possible mechanism for impaired placental leucine transfer in IUGR. The second objective of this study was to test this hypothesis by studying the uptake of leucine and lysine into isolated plasma membrane vesicles in the absence and presence of intravesicular glycine.
METHODS
Patients. Placentas from two groups of patients were studied. Control placentas were obtained from uneventful pregnancies, delivered vaginally or by cesarean section due to maternal indication. IUGR was defined as a birth weight below mean - 2 SD using intrauterine growth curves based on ultrasonically estimated fetal weight (18). IUGR babies were delivered vaginally or by means of cesarean section on fetal indication (signs of fetal distress). Estimated gestational age was determined from the last menstrual period and confirmed by ultrasound at 16-18 wk of gestation.
Preparation of syncytiotrophoblast plasma membranes. Placentas were placed on ice immediately after delivery, and the membrane isolation procedure was started within 1 h. MVM and BM vesicles were prepared simultaneously from the same placenta according to a well characterized protocol (19–21). Briefly, after initial homogenization and centrifugation steps (at 4°C, in 250 mM sucrose, 10 mM HEPES-Tris, pH 7.4), BM were separated from MVM by Mg2+ precipitation and further purified on a sucrose step gradient. Samples were snap frozen in liquid nitrogen and stored at -80°C. MVM and BM enrichment was assessed using standard activity assays for adenylate cyclase (22) and alkaline phosphatase (23). The production of cAMP by adenylate cyclase was measured by RIA (NEN Life Science Products, Boston, MA).
Measurements of L-lysine and L-leucine uptake. Uptake of L-lysine into vesicles was studied according the method described in detail by Eleno et al. (24). This protocol measures L-lysine influx in the presence of a membrane potential (inside negative) imposed by the presence of NaSCN. L-Leucine transport was measured as described previously (25) with some modifications. For both amino acids, vesicles were preloaded by incubation in 298 mM mannitol, 0.1 mM MgSO4, and 2 mM HEPES-Tris, pH 7.4 (buffer A), overnight at 4°C. Subsequently, vesicles were pelleted and resuspended in a small volume of the same buffer (protein concentration ∼5-10 mg/mL). Membrane vesicles were kept on ice until immediately before transport measurements when samples were warmed to 37°C. At time zero, 25 or 50 µL of vesicles were rapidly mixed (1:1) with the appropriate incubation buffer including 3H-L-lysine (0.2 µM) or 3H-L-leucine (0.5 µM). After 4-90 s, uptake of radiolabel was terminated by addition of 2 mL of ice-cold PBS, and vesicles were separated from the substrate medium by filtration on mixed ester filters (0.45-µm pore size, Millipore Corp., Bedford, MA) and washed with 6 mL of PBS. In lysine uptake experiments, incubation buffers included 104 mM NaSCN, 75 mM mannitol, 0.1 mM MgSO4, and 17 mM HEPES-Tris, pH 7.4. In experiments assessing the contribution of the y+ system 2 mM mannitol was exchanged with 2 mM L-glutamine (24). Nonmediated lysine flux was measured in the presence of 10 mM unlabeled substrate. In leucine transport experiments, NaSCN was replaced by NaCl in the incubation buffer. BCH in a final concentration of 20 mM was used to block transport mediated by the L system, and nonmediated flux was studied in the presence of 20 mM unlabeled L-leucine. In kinetic experiments, uptake of radiolabeled amino acids was measured in the presence of unlabeled L-lysine (4-1000 µM) or L-leucine (2-80 µM). Nonmediated fluxes (represented by uptakes in the presence of 10 mM L-lysine and 20 mM L-leucine, respectively) were subtracted before further analysis. Kinetic constants for lysine were estimated by fitting experimental data to the Michaelis-Menten equation for two saturable components (26), whereas kinetic parameters for leucine were obtained using the Eadie-Hofstee linear transformation of this equation (27).
In glycine trans-stimulation experiments, vesicles isolated only from uncomplicated pregnancies were used, and membrane vesicles from an individual placenta were divided into two parts. Vesicles were incubated overnight at 4°C in buffer A or buffer A with 2 mM glycine added. Subsequently, vesicles were pelleted and resuspended in buffer A without glycine. In all uptake experiments, each condition was studied in triplicate. Filters were dissolved in 2 mL of liquid scintillation fluid and counted. Uptake data were expressed as picomoles/mg of protein after subtracting appropriate blanks. The protein content of the vesicles was determined by the method of Bradford (28).
Statistics. Results are given as means ± SEM. Unpaired and paired t tests were used to evaluate data statistically. A p value < 0.05 was considered significant.
RESULTS
Selected clinical data are given in Table 1. The IUGR group included pregnancies with no other major complication. Fetal weight was 39% lower in the IUGR group and placental weight was reduced by 44%. IUGR fetuses had a lower ponderal index than control subjects (Table 1), suggesting asymmetric growth. In 4/6 cases, abnormal doppler blood flow patterns were registered in umbilical vessels during days or weeks before delivery. Furthermore, three IUGR fetuses were delivered by acute cesarean section due to signs of asphyxia (decreased CTG variability and tachycardia). The neonatal period was uneventful apart from hypoglycemia within 24 h of delivery in two neonates.
Enrichment of alkaline phosphatase activity in MVM was 18.4 ± 3.4 in the control group and 20.1 ± 7.8 in the IUGR samples. Enrichment of adenylate cyclase activity in BM was 19.5 ± 3.3 (control) and 21.0 ± 3.4 (IUGR). Enrichments in IUGR vesicles were not statistically different from those of control subjects. No adenylate cyclase activity was measurable in MVM, whereas alkaline phosphatase activity was enriched 2.9-fold in BM. At least in part, this is likely to be due to the presence of some alkaline phosphatase in the BM (29) rather than an indication of cross-contamination. Using immunoblotting and enzyme activity assays we have shown only little or no contamination by markers for mitochondria and endoplasmic reticulum in our plasma membrane fractions (30). These results confirm that MVM and BM with high enrichment and low contamination were obtained using this plasma membrane isolation procedure (19).
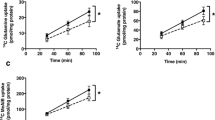
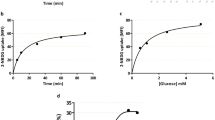
In initial studies we compared L-leucine and L-lysine uptakes in unfrozen vesicles (n = 3) with transport in vesicles that had been stored in -80°C for up to 6 mo (n = 3). We found that total and nonmediated uptakes were similar in these two groups, as well as the relative contribution of transport systems involved (data not shown). For all subsequent studies frozen vesicles were used. The uptake of labeled amino acids was rapid and reached equilibrium at 20-60 s (Fig. 1). Uptakes at 8 s were chosen to approximate initial rate. An "overshoot" was observed in L-lysine uptake curves, in particular in MVM (Fig. 1). This is likely due to the dissipation of the inside negative membrane potential imposed at time zero by the presence of NaSCN in the extravesicular buffer.
The contribution of nonmediated pathways to total transport of the charged L-lysine was small. In control vesicles nonmediated uptake represented 3 ± 1 and 9 ± 2% in MVM and BM, respectively (Fig. 2). In the presence of 2 mM L-glutamine, mediated lysine uptake in MVM was reduced by 29 ± 2%, representing the contribution of system y+L (24). The remaining 71 ± 2% corresponds to lysine flux mediated by system y+. In BM the relative contribution of the two transport systems to lysine transport was different (p < 0.05 versus MVM) in that the y+ system mediated 53 ± 4% and the y+L system 47 ± 4% of lysine uptake (Fig. 2). Lysine transport in MVM was not significantly altered in IUGR (Fig. 2). In contrast, IUGR was associated with a 44% (p < 0.05) lower mediated lysine uptake in BM compared with control vesicles.
Transport of L-lysine in MVM and BM isolated from control (n = 8) and IUGR placentas (n = 6). The contribution of the y+ and y+L systems was assessed in experiments in the presence of 2 mM L-glutamine. The fraction of mediated uptake inhibited by L-glutamine represents transport by the y+L system, whereas the uninhibited transport corresponds to y+. Non-mediated uptake was measured in the presence of unlabelled substrate. Means ± SEM. *p < 0.05 vs control.
Nonmediated uptake of the neutral amino acid L-leucine was substantial, comprising 23 ± 4% of total uptake in MVM and 30 ± 6% in BM control vesicles (Fig. 3). Leucine transport was mediated almost exlusively by the L system as demonstrated by the near complete inhibition of leucine uptake by BCH. In IUGR, leucine uptake was reduced both in MVM (-46%, p < 0.05) and in BM (-38%, p < 0.05). Kinetic constants were estimated in control and IUGR vesicles and are given in Table 2. These studies were not done for lysine uptake in MVM because we were unable to demonstrate any significant difference between groups in lysine uptakes in MVM at 8 s (Fig. 2). In all cases Km values were similar in control and IUGR groups (Table 2), indicating unaltered transporter affinity in IUGR. There was a trend toward 30-50% lower Vmax values in IUGR for both lysine and leucine transport; however, this difference was statistically significant only for lysine uptake by the y + L system in BM.
Transport of L-leucine in MVM and BM isolated from control (n = 8) and IUGR placentas (n = 6). Nonmediated uptake was measured in the presence of 20 mM of unlabeled substrate. BCH in a concentration of 10 mM was used to block transport mediated by the L system. In MVM control vesicles, mediated uptake of leucine in the presence of BCH (L inhibition) was 0 ± 0.003. Means ± SEM. *p < 0.05 vs control.
The presence of intravesicular glycine did not affect uptake of lysine (Fig. 4). In contrast, glycine stimulated leucine uptake by 98 ± 39% (p < 0.05, paired t test) in MVM. Intravesicular glycine did not significantly alter leucine uptake in BM.
DISCUSSION
Human fetuses with IUGR have reduced plasma concentrations of α-aminonitrogen, which to a large extent appears to be caused by lower concentrations of a number of essential amino acids (8,12,13). Fetal plasma concentrations of amino acids depend on placental transport but also other factors, such as fetal utilization of amino acids in anabolic and catabolic pathways. The activity of system A amino acid transporters has been shown to be reduced in the MVM of IUGR placentas (10,11). However, these findings do not provide a sufficient explanation for low fetal plasma levels of essential amino acids, which are not transported by system A.
The current study is the first report to demonstrate a reduction in the activity of transporters for essential amino acids in human placentas obtained from IUGR pregnancies. We found a lower mediated lysine uptake in BM vesicles as well as reductions in leucine transport both in MVM and BM. The kinetic data showed unaltered Km and a clear trend toward reduced Vmax, although statistically significant only for y+L-mediated lysine transport in BM. These findings are compatible with a reduction in the number of transporters. We suggest that the lower capacity to transport lysine and leucine of syncytiotrophoblast plasma membranes is likely to impair transplacental amino acid transport in IUGR pregnancies. It is possible that these changes play an important part in the pathophysiologic mechanisms resulting in altered growth pattern in this pregnancy complication.
A number of transport systems for amino acids have been reported to be present in the human placenta, but the mechanisms responsible for a net transplacental transport of amino acids from mother to fetus are not well characterized (14). This is particularly true for many of the essential amino acids, such as leucine and lysine. Despite severalfold higher concentrations of these amino acids in fetal compared with maternal plasma (15), suggesting an active transport, only sodium-independent transport systems appear to mediate lysine and leucine transport in human placenta (24–26,31). However, for cationic amino acids such as lysine the inside negative membrane potential present in most cells provides a driving force for accumulation into the cell (32). Human syncytiotrophoblast cells at term maintain a membrane potential that is approximately -21 mV (inside negative) across the MVM (33), and this electrical gradient will drive uptake of lysine into the syncytium against its concentration gradient. The mechanisms for net lysine transport across the BM into the fetal compartment is not well understood but might be related to unequal distribution of distinct transport systems for cationic amino acids to the two polarized plasma membranes of the syncytiotrophoblast cell (24,34). In MVM cationic amino acid uptake has been shown to be mediated by y+ and y+L systems (24,35), findings that are confirmed in the present study. Preliminary data in the study of Eleno et al. (24) suggested that the relative contribution of the y+L system to total lysine flux was substantially higher in BM than in MVM. Because this transporter was shown to be less sensitive to membrane potential than y+ and, in addition, was able to perform heteroexchange with neutral amino acids it was suggested that y+L could mediate net transport of lysine into the fetus across the BM (24). Under the conditions used in the present study the contribution of the y+L system to total lysine flux was 29% in MVM and 47% in BM, suggesting a polarized distribution of this transporter in the two syncytiotrophoblast plasma membranes. In addition to y+ and y+L systems, evidence for the presence of a third transport system for cationic amino acids in the BM has been provided by Furesz et al. (26) and Furesz and Smith (34). This system, b0,+, can exchange cationic for neutral amino acids and it has therefore been suggested to contribute to net lysine transport from the syncytiotrophoblast to the fetal circulation (34). The present study was not designed to evaluate possible contribution of b0,+ to overall lysine uptake. Consequently, the fraction of total lysine transport assigned to the y+ system may be overestimated in the BM.
The uptake of leucine mediated by system L can be influenced by the presence of other neutral amino acids as demonstrated by the pioneer work by Christensen (17,37) and Christensen et al. (36). In particular, a high intracellular concentration of another neutral amino acid could stimulate the uptake of leucine. We hypothesized that glycine might play a role in uphill transport of leucine in the human placenta. The basis for this hypothesis is that glycine is mainly transported into the syncytiotrophoblast cell by the sodium-dependent transport system A resulting in high intracellular concentrations (15). However, transfer of this amino acid to the fetus is limited (16), suggesting that much of the glycine taken up by the placenta diffuses back into the maternal circulation. Because glycine is also accepted by the L system, the outwardly directed glycine gradient might drive an uphill transport of other L system substrates, such as leucine, by trans-stimulation of the transporter. Our data show that the presence of glycine inside vesicles in physiologic concentrations markedly stimulated the uptake of leucine, but not lysine. Interestingly, glycine stimulated leucine uptake significantly only in MVM. This difference might provide the basis for a net transfer of leucine from the mother to the fetus. The underlying mechanism for these findings is unclear at present but may be related to the major differences in general membrane characteristics between these two plasma membranes. For example, the cholesterol content of BM is only 50% that of MVM (20) resulting in a high fluidity of the BM (21). The relationship between membrane composition in general, and membrane fluidity in particular, and the activity of transporters is well established (38). It is therefore possible that the affinity of the system L transporters for glycine is altered in the high fluidity environment in BM. Furthermore, the L system transporter might be present in two molecular isoforms with slightly different kinetics in the two plasma membranes. In IUGR, system A transporter activity in the placenta is reduced (10,11) and intracellular glycine concentrations in the placenta are therefore likely to be lower in this pregnancy complication. We speculate that a reduced glycine gradient in the placenta will impair leucine transport to the fetus and that this might be one additional mechanism causing lower fetal plasma concentrations of this essential amino acid in IUGR.
In the human placenta, the two polarized plasma membranes of the syncytiotrophoblast cell represent the main barrier for transplacental transport of amino acids. Therefore, characterization of transport activities of the individual membranes in vitro provides the basis for modeling nutrient fluxes in vivo. However, total delivery of amino acids to the fetus is influenced by a number of factors other than the activities of the individual transporters, such as placental amino acid metabolism, membrane potential and available exchange area. Little is known about possible alterations in placental metabolism or membrane potential in IUGR pregnancies. However, transport of the cationic amino acid lysine would be affected by changes in syncytiotrophoblast membrane potential. In a group of idiopathic IUGR placentas no major change in total trophoblastic surface area normalized to placental weight could be demonstrated (39). Furthermore, membrane composition as measured as the total phospholipid to protein ratio appears not to be altered in IUGR (9). These observations support the use of membrane protein as denominator in transport measurements to represent a crude measure of membrane area. Therefore it likely that the reduction in amino acid transporter activities reported in the current study represent a decreased transport also in vivo. However, our understanding of the transport functions of the IUGR placenta is incomplete and additional studies in isolated membranes, perfused placentas and the pregnant women are needed.
In the IUGR pregnancies included in this study no cause for the restricted growth, such as maternal hypertension, preeclampsia or diabetes with vasculopathy, was apparent. The evidence for asymmetric growth as well as abnormal umbilitical blood flow patterns and high incidence of intrapartum asphyxia indicate that these fetuses were compromised. Thus, they are likely to represent true growth restriction rather than genetically or constitutionally small babies. The altered growth pattern in IUGR can be regarded as an adaptation in response to suboptimal nutrient supply. The IUGR group in this study might represent a subgroup of growth restricted fetuses in which adaptation has been quite successful because they were carried to term. It is possible that alterations in the activity of placental amino acid and other nutrient transporters are even more pronounced in IUGR fetuses delivered earlier in gestation.
Abbreviations
- IUGR:
-
intrauterine growth restriction
- MVM:
-
microvillous membrane
- BM:
-
basal membrane
- BCH:
-
2-amino-2-norbornane-2-carboxylic acid
REFERENCES
Fanaroff AA, Wright LL, Stevenson DK, Shankaran S, Donovan, EF, Ehrenkranz RA, Younes N, Korones SB, Stoll BJ, Tyson JE, Bauer CR, Oh W, Lemons JA, Papile LA, Verter J 1995 Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991 through December 1992. Am J Obstet Gynecol 173: 1423–1431
Blair E, Stanley F 1990 Intrauterine growth and spastic cerebral palsy. I. Association with birth weight for gestational age. Am J Obstet Gynecol 162: 229–237
Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall C, Osmond C, Winter PD 1991 Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303: 1019–1022
Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS 1993 Fetal nutrition and cardiovascular disease in adult life. Lancet 341: 938–941
Hay WW, Catz CS, Grave GD, Yaffe SJ 1997 Workshop summary: Fetal growth: its regulation and disorders Pediatrics 99: 585–591
Economides DL, Nicolaides KH 1989 Blood glucose and oxygen tension in small-for-gestational-age fetuses. Am J Obstet Gynecol 160: 1091–1094
Soothill PW, Nicolaides KH, Campbell S 1987 Prenatal asphyxia, hyperlacticaemia, hypoglycaemia and erythroblastosis in growth retarded fetuses. BMJ 294: 1051–1053
Cetin I, Marconi AM, Bozzetti P, Sereni, LP, Corbetta, C, Pardi, G, Battaglia, FC 1988 Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol 158: 120–126
Jansson T, Wennergren M, Illsley NP 1993 Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 77: 1554–1562
Dicke JM, Henderson GI 1988 Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci 295: 223–227
Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RDH, Sibley CP 1993 Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34: 661–665
Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC 1990 Umbilical amino acid concentrations in normal and growth retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162: 253–261
Economides DL, Nicolaides KH, Gahl WA, Bernadini I, Evans MI 1989 Plasma amino acids in appropriate- and small-for-gestational-age fetus. Am J Obstet Gynecol 161: 1219–1227
Moe AJ 1995 Placental amino acid transport. Am J Physiol 268: C1321–C1331
Yudilevich DL, Sweiry JH 1985 Transport of amino acids in the placenta. Biochim Biophys Acta 822: 169–201
Cetin I, Marconi AM, Baggiani AM, Buscaglia M, Pardi G, Fennessey PV, Battaglia FC 1995 In vivo placental transport of glycine and leucine in human pregnancies. Pediatr Res 37: 571–575
Christensen HN 1975 Biological Transport, 2nd Ed. WA Benjamin, Reading, MA, 380–382.
Marsál K, Persson P-H, Larsen T, Lilja H, Selbing A, Sultan B 1996 Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 85: 843–848
Illsley N P, Wang Z Q, Gray A, Sellers M C, Jacobs M M 1990 Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta 1029: 218–226
Jansson T, Powell TL, Illsley, NP 1993 Non-electrolyte solute permeabilities of human placental microvillous and basal membranes. J Physiol 468: 261–274
Jansson T, Illsley, NP 1993 Osmotic water permeabilities of human placental microvillous and basal membranes. J Membrane Biol 132: 147–155
Schultz G, Jakobs KH 1984 Adenylate cyclase. In: Bergmeyer H (ed) Methods of Enzymatic Analysis, Vol. IV. Enzymes 2: Esterases, Glycosidases, Lyases, Ligases. Verlag Chemie, Weinham, Germany, 369–378.
Bowers, G, McComb, R 1966 A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin Chem 12: 70–89
Eleno N, Devés R, Boyd CAR 1994 Membrane potential dependence of the kinetics of cationic amino acid transport systems in human placenta. J Physiol 479: 291–300
Johnson LW, Smith CH 1988 Neutral amino acid transport systems of microvillous membrane of human placenta. Am J Physiol 254: C773–C780
Furesz TC, Moe AJ, Smith CH 1991 Two cationic amino acid transport systems in human placental basal membranes. Am J Physiol 261: C246–C252
Hofstee BHJ 1959 Non-inverted versus inverted plots in enzyme kinetics. Nature 184: 1296–1298
Bradford MM 1976 A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Jones C, Fox H 1976 An ultrahistochemical study of the distribution of acid and alkaline phosphatases in placentae from normal and complicated pregnancies. J Pathol 118: 143–151
Powell TL, Lundquist C, Doughty IM, Glazier JD, Jansson T 1998 Mechanisms of chloride transport across the syncytiotrophoblast basal membrane in the human placenta. Placenta 19: 315–321
Hoeltzli SD, Kelley LK, Moe AJ, Smith CH 1990 Anionic amino acid transport systems in isolated basal plasma membrane of human placenta. Am J Physiol 259: C47–C55
White MF 1985 The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta 822: 355–374
Birdsey TJ, Boyd RDH, Sibley CP, Greenwood SL 1997 Microvillous membrane potential (Em) in villi from first trimester human placenta: comparison to Em at term. Am J Physiol 273: R1519–R1528
Furesz TC, Smith CH 1997 Identification of two leucine-sensitive lysine transport activities in human placental basal membrane. Placenta 18: 649–655
Furesz TC, Moe AJ, Smith CH 1995 Lysine uptake by human placental microvillous membrane: comparison of system y + with basal membrane. Am J Physiol 268: C755–C761
Christensen HN, Streicher JA, Elbinger RL 1948 Effects of feeding individual amino acids upon the distribution of other amino acids between cells and extracellular fluid. J Biol Chem 172: 515–524
Christensen HN 1985 On the strategy of kinetic discrimination of amino acid transport systems. J Membr Biol 84: 97–103
Molitoris B A 1987 Membrane fluidity: measurement and relationship to solute transport. Semin Nephrol 7: 61–71
Teasdale F, Jean-Jacques G 1988 Intrauterine growth retardation: morphometry of the microvillous membrane of the human placenta. Placenta 9: 47–55
Author information
Authors and Affiliations
Additional information
Supported by Swedish Medical Research Council (10838, 11834, and 02591), the Axel and Margaret Ax:son Johnson Foundation, the Emil and Vera Cornell Foundation, the Bank of Sweden Tercentenary Foundation, Frimurare-Barnhus-direktionen, the Swedish Society for Medical Research, the Åhlens Foundation, the Lars Hierta Foundation, the Samariten Foundation, the Sven Jerring Foundation, the General Maternity Hospital Foundation, the Magnus Bergvall Foundation, the Craaford Foundation and the Willhelm and Martina Lundgrens Foundation.
A preliminary account of this study was presented at the 44th Annual Meeting of the Society for Gynecological Investigation, San Diego, CA, March 19-22, 1997.
Rights and permissions
About this article
Cite this article
Jansson, T., Scholtbach, V. & Powell, T. Placental Transport of Leucine and Lysine Is Reduced in Intrauterine Growth Restriction. Pediatr Res 44, 532–537 (1998). https://doi.org/10.1203/00006450-199810000-00011
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199810000-00011
This article is cited by
-
CHOP upregulation and dysregulation of the mature form of the SNAT2 amino acid transporter in the placentas from small for gestational age newborns
Cell Communication and Signaling (2023)
-
Fetal Sex Does Not Impact Placental Blood Flow or Placental Amino Acid Transfer in Late Gestation Pregnant Sheep With or Without Placental Insufficiency
Reproductive Sciences (2022)
-
Sexually dimorphic patterns in maternal circulating microRNAs in pregnancies complicated by fetal growth restriction
Biology of Sex Differences (2021)
-
Human placental uptake of glutamine and glutamate is reduced in fetal growth restriction
Scientific Reports (2020)
-
Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies
Angiogenesis (2020)