Abstract
A major route of tryptophan metabolism is via the hepatic and cerebral synthesis of kynurenine, a substance subsequently used by astrocytes in the brain for the production of the neuroactive substances kynurenic acid and quinolinic acid. Both kynurenic and quinolinic acids have been implicated in modulating the activity of excitatory amino acid pathways in the brain, the former as a neuroprotectant because of its antagonist properties, and the latter as an excitotoxin because of its agonist actions, at NMDA receptors. We therefore determined the concentrations of tryptophan and kynurenine in maternal venous and umbilical cord blood, and in amniotic fluid, of infants after labor and vaginal delivery, and after delivery by cesarean section. Concentrations of tryptophan and kynurenine were significantly higher in umbilical vein plasma compared with maternal venous plasma. Tryptophan and kynurenine concentrations in umbilical vein plasma and amniotic fluid were significantly higher after labor, compared with samples obtained from infants of the same gestational age delivered by cesarean section. There was no umbilical vein-to-artery concentration difference for kynurenine in samples obtained after either labor or cesarean section, but there was a significant gradient for tryptophan in samples obtained after vaginal delivery, indicating increased transfer of this amino acid during labor. There was a significant correlation between umbilical vein tryptophan and kynurenine concentrations for both the labor and cesarean section groups, and plasma kynurenine concentrations were also significantly correlated with both umbilical vein cortisol concentrations and the duration of the second stage of labor in the vaginally delivered infants. These results suggest that the placental transfer of tryptophan and the fetal synthesis of kynurenine are increased during labor. These findings have implications for understanding the vulnerability of the infant brain to ischemic/hypoxic damage in the perinatal period. By analogy with the adult brain, the molar ratio of these substances is likely to determine the susceptibility of the brain to seizure and excitotoxic damage.
Similar content being viewed by others
Main
In man, the essential amino acid tryptophan is metabolized by two major pathways, through either the kynurenine or indoleamine pathways(1,2), in addition to its incorporation into protein. Kynurenine is the immediate precursor of kynurenic acid, an endogenous antagonist at NMDA-receptor pathways in the brain and also of quinolinic acid, an agonist at NMDA receptors(3). Both substances are synthesized by astrocytes in the brain and released into the extracellular space in a molar ratio of approximately 5:1 favoring the antagonist kynurenic acid(4).
L-Kynurenine is selectively transported across the blood-brain barrier by the large neutral amino acid carrier system(5) and most(but not all)(6,7) of the kynurenine metabolites in the brain are derived from the peripheral pool of kynurenine. Tryptophan also enters the brain via the amino acid transport system where it is used for synthesis of indoleamines, and for the synthesis of both kynurenic acid and quinolinic acid from the common precursor, kynurenine(6–8).
Little is known about the metabolic fate of tryptophan in fetal life, particularly with respect to kynurenine synthesis, but it is reasonable to suspect from studies in adult animals that this pathway could influence activity in many regions of the brain, particularly after episodes of hypoxia, whether acute or chronic in duration. Much recent evidence has implicated excitatory amino acids in the neural damage which follows hypoxiaischemia, hypoglycemia, and cerebral hypoperfusion(9,10). Kynurenic acid has been shown to be neuroprotective because of its antagonist actions at the glycine binding site of the receptors for aspartate and glutamate(3). The action of kynurenic acid is to minimize the influx of calcium into the postsynaptic neurones, and therefore to decrease the likelihood and extent of neural damage which follows the excessive accumulation of calcium in the neuron. On the other hand, increase of quinolinic acid synthesis would dispose the brain to excitotoxic damage because of the agonist effect of this substance at NMDA receptors(11). The supply of tryptophan and its conversion to kynurenine might therefore determine the relative abundance of these two important metabolites in the fetal brain during the hypoxic and hypoglycemic conditions which may be present before, or which develop during, the birth process.
Kynurenine is formed via N-formylkynurenine by the action of TDO, an enzyme with a high substrate specificity for trytophan and which is found mainly in the liver(12), but also in the brain(6). Indolyl 2,3-dioxygenase is a more widely distributed enzyme, which may catalyze the formation of kynurenine, and is present in macrophages, microglia, and in the human placenta(13,14). However, the greater part of kynurenine found in the body is derived from the action of hepatic TDO. This enzyme is unsaturated at physiologic concentrations of tryptophan, and is up-regulated by high substrate concentrations(15) and by corticosteroids(16,17). Because the fetal secretion of cortisol increases in late gestation, and is increased further by any of several factors including hypoxia, hypercapnia, hypoglycemia, and labor(18–22), it is possible that the formation of kynurenine is increased toward term, and particularly at the time of birth, thereby providing the fetal brain with an increased level of substrate for the production of either kynurenic or quinolinic acid, or both.
To test this proposition, we measured tryptophan and kynurenine concentrations in maternal and umbilical cord plasma samples obtained from women who delivered infants at term after a normal labor, or after delivery by elective cesarean section at term before labor had commenced. We hypothesized that kynurenine concentrations would be higher in the samples obtained after labor compared with those from the cesarean deliveries. The results are in agreement with this proposal, but unexpectedly, we also found that umbilical vein concentrations of trytophan were higher after labor. The results suggest that the formation of kynurenine in the fetus is closely coupled to the prevailing concentration of tryptophan, which is in turn increased in the fetal circulation by mechanisms associated with normal labor and delivery.
METHODS
Samples of maternal venous blood, umbilical vein or artery blood, and amniotic fluid were collected from patients after either normal labor followed by vaginal delivery, or after elective cesarean section before labor had commenced. The study had received prior approval from the Human Research and Ethics Committee of the Monash Medical Centre. Each patient received a written explanation and signed a consent form before being recruited to the study.
First, paired samples of maternal venous and umbilical vein blood were collected from 4 patients after labor and vaginal delivery, and from 15 patients after elective cesarean section. In a second group, paired samples of umbilical vein and artery blood were collected from 6 patients after normal labor and vaginal delivery, and from 10 patients after elective cesarean section. Third, paired samples of umbilical vein blood and amniotic fluid were collected from 13 patients after normal vaginal delivery, and from 12 patients after elective cesarean section. Detailed obstetric information for these patients, including gestational age, birth weight, and Apgar scores was available from the medical summary sheets.
Maternal blood was obtained from an antecubital vein. Blood was collected from the umbilical cord between clamps after the complete delivery of the baby. When samples were obtained from an umbilical artery and vein they were withdrawn within 1 min of each other. The blood was drawn into a 5-mL syringe containing a drop of heparin. An aliquot of the umbilical vein blood was used immediately to measure blood gases, pH, and electrolytes using a calibrated blood gas analyzer (ABL 500, Radiometer, Copenhagen, Denmark). Blood was transferred into a plastic, capped tube and kept on ice until the plasma was recovered after centrifugation at 1000 × g for 10 min in a refrigerated centrifuge. Amniotic fluid was collected as soon as possible after rupture of the membranes in the labor group, or after the baby had been removed from the uterus in the cesarean section group. The amniotic fluid and plasma were kept at -20°C until analysis of tryptophan and kynurenine.
Analyses. Tryptophan and kynurenine were analyzed using HPLC. Samples were deproteinized with an equal volume of 10% trichloroacetic acid, and the supernatant was recovered after centrifugation at 2000 ×g for 10 min at 4°C. Aliquots (50 or 100 µL) of the supernatant were injected onto a 3.9 × 300-mm C18 silicaµBondapak column (Waters Associates, Milford, MA) using a programmable autoinjector (WISP, Waters Associates). The mobile phase consisted of a 40 mM sodium acetate/citric acid buffer solution in 5% acetonitrile; the buffer solution was adjusted to pH 5 with 4 M KOH before adding the acetonitrile(23). The flow rate of the mobile phase was 0.7 mL/min. Tryptophan and kynurenine were detected by UV absorption at 278 and 363 nm, respectively. Under these conditions the elution times of kynurenine and tryptophan were 10.2 and 17.2 min, respectively. The peaks were displayed on a chart recorder using a paper speed of 0.5 cm/min. Sensitivity of the assay was approximately 25 pmol for both analytes, and the intraassay coefficient of variation was 2.9 ± 0.2% and 2.7 ± 0.4% for tryptophan and kynurenine, respectively. The interassay coefficient of variation was 12.7 ± 3.1%. A complete set of standards for both analytes was always included with every set of samples run on a particular day.
An aliquot of umbilical vein plasma (200 µL) was used to measure cortisol concentrations by RIA. The sensitivity of the assay was 0.8 ng/mL, and the intraassay coefficient of variation was 10.1%.
Statistics. Results are presented as mean ± SEM, except for Apgar scores which are presented as the mode and range. The unpaired t test was used to compare values between the labor and cesarean section groups. When the variances of groups to be compared were significantly different, the data were transformed (square root, log10, and so forth), before calculating the t value. Association between variables was assessed by computing the Pearson correlation coefficient. p < 0.05 was considered to be statistically significant.
RESULTS
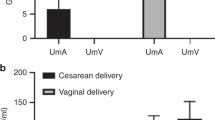
Comparison of paired samples of maternal and umbilical vein plasma revealed that both tryptophan and kynurenine were present in significantly higher concentrations in the umbilical vein after both cesarean section and normal labor (Fig. 1). Moreover, the umbilical vein concentration of both substances was higher after labor, compared with cesarean section. In contrast, the concentrations of tryptophan and kynurenine in maternal blood were similar in both groups of patients(Fig. 1).
Tryptophan and kynurenine was also measured in paired samples obtained from the umbilical artery and vein after either cesarean section (n= 10) or normal vaginal deliveries (n = 6). In both groups the mean venoarterial concentration difference for kynurenine (cesarean section, 0.4± 0.8 µM; labor, 0.6 ± 1.1 µM) was not significantly different from zero. The vein-to-artery concentration difference for tryptophan was also not different from zero for the cesarean section group(5.6 ± 7.1 µM), but it was significantly greater than zero in the samples obtained after labor (37.2 ± 14.3 µM; p < 0.05).
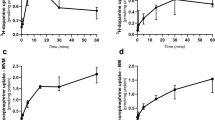
Umbilical vein blood and amniotic fluid samples were then collected form a further 25 patients (13 labor, 12 cesarean section) for whom detailed obstetric history and delivery details were available. Mean birth weight and length of gestation were not different for the two groups(Table 1). There was also no difference between the groups for umbilical vein blood gases, pH, blood Na+ and K+, bicarbonate, and absolute base excess. The modal 1 and 5-min Apgar scores were not different for the infants from each group. In these patients the umbilical vein plasma tryptophan, kynurenine, and cortisol concentrations were significantly higher after labor compared with those from cesarean section(Table 1). There was a significant correlation between tryptophan and kynurenine in umbilical vein plasma for both the labor(r = 0.83; p < 0.02) and cesarean section (r= 0.65; p < 0.02) groups (Fig. 2); the slope and intercept coefficients derived from the regression equations for the two groups were not significantly different, and the data were therefore combined to yield a correlation between tryptophan and kynurenine concentrations in umbilical vein plasma of 0.91 (p < 0.01). Kynurenine concentrations in umbilical vein plasma were also significantly correlated with the length of the second stage of labor (Fig. 3); there was no association between the length of labor and tryptophan concentrations. The concentrations of umbilical vein tryptophan and kynurenine were not correlated with blood gases or pH, but there was a significant correlation between kynurenine and cortisol concentrations r = 0.59,p < 0.05) when the data from the two groups were combined.
In amniotic fluid, the concentrations of tryptophan and kynurenine were significantly higher after labor compared with cesarean section(Fig. 4). There was no correlation between the concentrations of tryptophan and kynurenine in amniotic fluid, or between each of these substances and the blood gases, pH, and electrolyte values obtained from the umbilical blood samples.
DISCUSSION
A significant finding of this study is the higher concentrations of tryptophan and kynurenine found in umbilical vein blood after normal labor with vaginal delivery, compared with cesarean section. The length of gestation was comparable in the two groups, as were the blood gases and the acid-base status, so neither gestational age nor fetal condition would appear to account for the differences. The higher concentration of tryptophan in particular is surprising given that this essential amino acid is derived entirely from maternal diet, and passes across the placenta by a large neutral (or system 1) amino acid transport(24,25). It might have been expected that active transport mechanisms within the placenta become less efficient during labor, particularly as the placenta begins to separate from the wall of the uterus, and if hypoxia and acidosis develop as a consequence. However, the concentration difference for tryptophan between the umbilical vein and artery after vaginal delivery was significant, suggesting that there is increased transfer of this amino acid during labor. There may also be a placental pool of tryptophan which continues to pass into the fetal circulation despite the gradual deterioration of maternal-to-fetal transport.
It is not known if kynurenine crosses the placenta from mother to fetus. Fetal concentrations were higher than maternal, but no significant concentration difference was found between the umbilical vein and artery, confirming a previous report(26). Although it would seem likely that kynurenine could be transported across the placenta by facilitated diffusion as it is across the blood-brain barrier(5), the results suggest that fetal plasma kynurenine arises from hepatic conversion of tryptophan, rather than by synthesis in the placenta by the action of either placental TDO, or by the previously identified indolyl 2,3-dioxygenase-like enzyme in the placenta(14).
There was a close correlation between tryptophan and kynurenine concentrations in the umbilical vein samples, consistent with the substrate-product relationship of the two substances. The higher concentrations of kynurenine in the fetal, compared with the maternal, circulation suggests that the rate of synthesis by the fetus is higher. The activity of hepatic TDO is increased by both high substrate concentration(15) and by cortisol(16). We have recently shown that fetal sheep have a considerably greater ability to convert tryptophan to kynurenine compared with the mother, a function which probably depends on the induction of hepatic enzyme by tryptophan in the presence of cortisol (I. Nitsos and D. Walker, unpublished observations). Thus, the increase of kynurenine in the umbilical vein at birth after normal labor may be the result of both the increased availability of tryptophan and the higher cortisol concentrations which are present at this time.
The concentrations of both tryptophan and kynurenine were also elevated in amniotic fluid after labor. In the adult kidney amino acids are almost completely reabsorbed by the proximal tubule(27), but in the fetus the tubular resorption of amino acids may be less efficient. There was no correlation between the concentrations of either tryptophan or kynurenine in amniotic fluid and umbilical plasma, suggesting that the renal clearance of these substances is not directly related to their rates of glomerular filtration. There must be further clearance from the amniotic sac, but whether this occurs into the maternal circulation across the membranes, or is by uptake into the fetal circulation after swallowing of amniotic fluid, is not known.
Tryptophan and kynurenine enter the brain by means of a large neutral amino acid transporter(5). Both substances contribute to cerebral production of kynurenic and quinolinic acids as the brain also contains TDO and has the capacity to convert tryptophan to kynurenine(6,7). Both hepatic TDO and the enzymes involved in kynurenine metabolism in the brain do not appear to be saturated at physiologic concentrations of their substrates(2,8,28). Thus, it is reasonable to suggest that the production of kynurenic and/or quinolinic acid by the fetal brain could be higher during and after a normal labor when the two substrates are present in higher concentrations in the fetal circulation. Whether kynurenic or quinolinic acids actually reach extracellular concentrations which could have significant effects on glutamate receptors mechanisms needs to be established. In adult animals peripheral administration of either tryptophan(29) or kynurenine(28–30) result in the increase of both kynurenic and quinolinic acids in extracellular brain fluid and cerebrospinal fluid, but the amount of each substance administered was large and the plasma concentrations are likely to be greatly in excess of those measured in this study. However, it is interesting to note that these peripheral loading experiments resulted in a much greater and more prolonged increase of quinolinic acid(28,29), a result if it were to occur in the fetus would increase the risk of excitotoxic damage to the brain.
The extent to which the synthesis of either quinolinic or kynurenic acid is increased in the fetal brain when the availability of tryptophan and kynurenine is increased is presently unknown. In rats, resting concentrations of kynurenine and kynurenic acid are 3-5 times higher in the brain prenatally, and gradually decline after birth to adult values(31). The developmental profile of quinolinic acid in the brain is not known. Preferential synthesis of kynurenic acid might afford some degree of neuroprotection against the risk of cerebral damage which is a consequence of the asphyxia present at some time during most vaginal deliveries, and which may become extreme when delivery is obstructed or prolonged. In adult and newborn animals the neuroprotective function of kynurenic acid has been demonstrated only after infusions of relatively large doses of either kynurenic acid itself(32), or the peripheral administration of kynurenine either alone(33) or in combination with probenecid to block efflux of kynurenic acid from the brain(30). However, the synthesis of quinolinic acid, which has been implicated in the cytotoxic damage that follows cerebral hypoxia/ischemia(13,34,35), assumes greater importance with the demonstration that endogenous kynurenic acid ameliorates the extent of damage caused by this glutamate receptor agonist(36). In adult mice(37) and rats(29) systemic loading with tryptophan results in the increase of quinolinic acid in the brain to potentially neurotoxic levels. The capacity of the fetal brain to metabolize tryptophan and kynurenine to these neuroactive end-products is not known, and may differ from the adult brain. It is clearly important to understand the extent to which these two kynurenine metabolites are produced in the fetal brain and how this depends on the peripheral availability of the substrates, particularly at the end of gestation and during parturition when cerebral energy levels and oxygenation may be reduced.
Abbreviations
- NMDA:
-
N-methyl-D-aspartate
- TDO:
-
tryptophan 2,3-dioxygenase
References
Huether G, Hajak G, Reuner A, Poeggeler B, Blomer M, Rodenbeck A, Ruther E 1992 The metabolic fate of infused L-tryptophan in men: possible clinical implications of the accumulation of circulating tryptophan and tryptophan metabolites. Psycopharmacology 109: 422–432.
Peters JC 1991 Tryptophan nutrition and metabolism: an overview. In: Schwarcz, R (ed) Kynurenine and Serotonin Pathways. Plenum Press, New York
Stone TW 1993 Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev 45: 310–379.
Guidetti P, Eastman CL, Schwarcz R 1995 Metabolism of[5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J Neurochem 65: 2621–2632.
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR 1991 Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56: 2007–2017.
Gal EM 1974 Cerebral tryptophan 2,3-dioxygenase(pyrrolase) and its induction in the rat brain. J Neurochem 22: 861–863.
Gal EM, Sherman AD 1978 Synthesis and metabolism of L-kynurenine in rat brain. J Neurochem 30: 607–613.
Bender DA 1989 The Kynurenine pathway of tryptophan metabolism. In: Stone TW (ed) Quinolinic Acid and the Kynurenines. CRC Press, Boca Raton, FL, pp 5–28.
Choi DW, Rothman SM 1990 The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 13: 171–182.
Meldrum B, Evans M, Griffith, Simon R 1985 Ischaemic brain damage: the role of excitatory activity and calcium entry. Br J Anaesth 57: 44–46.
Stone TW, Perkin MN 1981 Quinolinic acid, a potent endogenous excitant at amino acid receptors in the CNS. Eur J Pharmacol 72: 411–412.
Miller LL 1962 The role of the liver and the non-hepatic tissues in the regulation of free amino acid levels in the blood. In: Holden J (ed) Amino Acid Pool. Elsevier, New York, pp 708–721.
Heyes MP 1993 Metabolism and neuropathologic significance of quinolinic acid and kynurenic acid. Biochem Soc Trans 21: 83–89.
Yamazaki F, Kuroiwa T, Takikawa O, Kido R 1985 Human indolylamine 2,3-dioxygenase. Biochem J 230: 635–638.
Knox WE, Mehler AH 1951 The adaptive increase of the tryptophan peroxidase-oxidase system of the liver. Science 113: 237–240.
Joseph MH, Young SN, Curzon G 1976 The metabolism of a tryptophan load in rat brain and liver. The influence of hydrocortisone and allopurinol. Biochem Pharmacol 25: 2599–2604.
Schimke RT, Sweeney EW, Berlin CM 1964 An analysis of the kinetics of rat liver tryptophan pyrrolase induction: the significance of both enzyme synthesis and degradation. Biochem Biophys Res Commun 15: 214–219.
Clapp JH III, Auletta FJ, Farnham J, Larrow RW, Mann LI 1982 The ovine fetoplacental endocrine response to placental damage. Am J Obstet Gynecol 144: 47–54.
Martinsen K, Peltola J, Tervila L, Virtanen A 1982 Umbilical cord cortisol and arterial pH levels in spontaneous and induced labors. Obstet Gynecol 59: 171–175.
Parker CR, Favor JK, Carden LC, Brown CH 1993 Effects of intrapartum stress on fetal adrenal function. Am J Obstet Gynecol 169: 1407–1411.
Ramin SM, Porter JC, Gilstrap LC, Rosenfeld CR 1991 Stress hormones and acid-base status of human fetuses at delivery. J Clin Endocrinol Metab 73: 182–186.
Ruth V, Hallman M, Laatikainen T 1993 Corticotropin-releasing hormone and cortisol in cord plasma in relation to gestational age, labor and fetal distress. Am J Perinatol 10: 115–117.
Krstulovic AM, Friedman MJ, Colin H, Guiochon G, Gaspar M, Pajer KA 1984 Analytical methodology for assays of tryptophan metabolites in control subjects and newly abstinent alcoholics: preliminary investigation by liquid chromatography with amperometric detection. J Chromatogr 297: 271–281.
Boyd R, Kudo Y 1994 Transport functions of the placenta and fetal membranes. In: Thorburn GD, Harding R (eds) Textbook of Fetal Physiology. Oxford University Press, New York, pp 6–15.
Ganapathy ME, Leibach FH, Mahesh VB, Howard JC, Devoe LD, Ganapathy V 1986 Characterisation of tryptophan transport in human placental brush-border membrane vesicles. Biochem J 238: 201–208.
Morita I, Kawamoto M, Yoshida H 1992 Difference in the concentration of tryptophan metabolites between maternal and umbilical foetal blood. J Chromatogr 576: 334–339.
Vander AJ 1995 Renal Physiology, 3rd Ed. McGraw-Hill, New York, pp 58–59.
Jausch DA, Sethy VH, Weick BG, Chase TH, Schwarcz R 1993 Intravenous administration of L-kynurenine to rhesus monkeys: effects on quinolate and kynurenate levels in serum and cerebrospinal fluid. Neuropharmacology 32: 467–472.
Miller J, MacGarvey V, Beal MF 1992 The effect of peripheral loading with kynurenine and probenecid on extracellular striatal kynurenic acid concentrations. Neurosci Lett 146: 115–118.
Vecsai L, Miller J, MacGarvey U, Beal MF 1992 Effects of kynurenine and probenecid on plasma and brain tissue concentrations of kynurenic acid. Neurodegeneration 1: 17–26.
Beal MF, Swartz KJ, Isacson O 1992 Developmental changes in brain kynurenic acid concentrations. Dev Brain Res 68: 136–139.
Andine P, Lehmann A, Ellren K, Wennberg E, Kjellmer I, Nielsen T, Hagberg H 1988 The excitatory amino acid antagonist kynurenic acid administered after hypoxic-ischemia in neonatal rats offers neuroprotection. Neurosci Lett 90: 208–212.
Nozaki K, Beal MF 1992 Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J Cereb Blood Flow Metab 12: 400–407.
Schwarcz R, Du F 1991 Quinolinic acid and kynurenic acid in the mammalian brain. In: Schwarcz R, Young SN, Brown RR (eds) Kynurenine and Serotonin Pathways: Progress in Tryptophan Research. Plenum Press, New York, pp 185–199.
Schwarcz R, Whetsell WO, Mangano RM 1983 Quinolinic acid: an endogenous metabolite that causes axon-sparing lesions in rat brain. Science 218: 316–318.
Foster AC, Vezzami A, French D, Schwarcz R 1984 Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related metabolite quinolinic acid. Neurosci Lett 48: 273–278.
Reinhard JF, Flanagan EM, Madge DJ, Iyer R, Salter M 1996 Effects of 540C91[(E)-3-[2-(4′-pyridyl)-vinyl]-1H-indole], an inhibitor of hepatic tryptophan dioxygenase, on brain quinolinic acid in mice. Biochem Pharmacol 51: 159–163.
Acknowledgements
The authors thank the medical and nursing staff of the Labour Ward, Monash Medical Centre, for their assistance in obtaining the maternal, umbilical vein, and amniotic fluid samples, and to Professor Shaun Brennecke and Dr. Greg Rice (Perinatal Research Centre, Royal Women's Hospital, Carlton, Victoria) for making available the umbilical vein and artery samples.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kazda, H., Taylor, N., Healy, D. et al. Maternal, Umbilical, and Amniotic Fluid Concentrations of Tryptophan and Kynurenine after Labor or Cesarean Section. Pediatr Res 44, 368–373 (1998). https://doi.org/10.1203/00006450-199809000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199809000-00017
This article is cited by
-
Differential Distribution of Tryptophan-Metabolites in Fetal and Maternal Circulations During Normotensive and Preeclamptic Pregnancies
Reproductive Sciences (2022)
-
Potato- An Important Source of Nutritional Kynurenic Acid
Plant Foods for Human Nutrition (2012)
-
Presence of kynurenic acid in food and honeybee products
Amino Acids (2009)
-
Endogenous kynurenines as targets for drug discovery and development
Nature Reviews Drug Discovery (2002)