Abstract
Taurine is an essential amino acid during fetal life and appears to be vital for the growth of the fetus and for the development of the CNS. In intrauterine growth restriction (IUGR), fetal plasma concentrations of taurine are reduced, and we tested the hypothesis that this is caused by altered placental transport of taurine. Syncytiotrophoblast microvillous membrane (MVM) and basal membrane (BM) vesicles were isolated from control(fetal weight, 3068 ± 191 g; gestational age, 37.0 ± 0.7 wk; n = 13) and IUGR pregnancies (fetal weight, 1724 ± 118 g; gestational age, 35.8 ± 0.7 wk; n = 11). Uptake of[3H]taurine (0.5 µM) was studied at 22°C using rapid filtration techniques. Sodium stimulated taurine uptake 35-fold in MVM, confirming Na+-dependent transport in this membrane. A Na+-dependent taurine transport could also be demonstrated in BM; however, the activity was only 6% of that in MVM. Na+-independent transport activities were similar in MVM and BM. In IUGR, MVM Na+-dependent taurine transport was reduced by 34% (p < 0.05), whereas Na+-independent uptake was unaltered. In contrast to MVM, Na+-dependent taurine uptake in BM was unaffected by IUGR, whereas Na+-independent transport was decreased by 33% (p < 0.05). The highly polarized distribution of the Na+/taurine cotransporter to the MVM in conjunction with similar Na+-independent transport rates for taurine in MVM and BM provides the basis for net taurine flux from the mother to the fetus. These data suggest that the low plasma concentrations of taurine in IUGR fetuses are caused by a reduced activity of placental taurine transporters.
Similar content being viewed by others
Main
IUGR is a pregnancy complication associated with adverse outcomes such as neurodevelopmental handicaps(1,2). In addition, restricted intrauterine growth has been implicated as a risk factor for disease in adult age(3,4). Placental insufficiency is a concept used clinically to indicate that impaired placental transport functions and blood flow are believed to be the cause of the altered growth pattern in many cases of IUGR. The activity of the system A amino acid transporter has been shown to be reduced in MVMs isolated from placentas of small for gestational age babies(5,6). However, whether other transport functions might be altered in IUGR remains to be established.
Taurine (2-aminoethanesulfonic acid) is a β-amino acid that is not incorporated into proteins. A multitude of important physiologic functions of taurine have been suggested. For example, taurine appears to be a neuromodulator(7) and is important in the cellular response to hypo-osmotic challenge(8). The main dietary sources of taurine are meat and fish; however, taurine can also be synthesized from cysteine in humans. Cats, who are strictly carnivorous, have lost their ability to synthesize taurine, and cats fed on a taurine-deficient diet suffer from retinal damages(9). The crucial importance of taurine for normal fetal development became apparent when pregnant cats were fed a semisynthetic diet lacking taurine, which resulted in abortion or delivery of growth-retarded kittens(10). The growth of the brain, especially the cerebellum, was retarded in these live-born kittens, and they showed signs of impaired neurologic function(11).
Taurine can be regarded as an essential amino acid during fetal life, because the capacity to synthesize taurine is low or absent in the human fetus(12,13). Consequently, the fetus is dependent on placental transport for a continuous supply of this important amino acid. In the human placenta, the transport of taurine includes uptake from maternal blood by transfer across the syncytiotrophoblast MVM and subsequently transport to the fetus across the BM. The tissue concentrations of taurine in human placenta are 100-200-fold higher than the concentration in maternal blood(14), indicating the presence of a highly efficient active transport of taurine in the MVM. The transporter protein in the MVM has been studied thoroughly(15–18). It is a high affinity Na+- and Cl--dependent transporter system, specific for β-amino acids, which transports Na+, Cl-, and taurine in a 2:1:1 ratio. The human placental taurine transporter has been cloned and characterized(19). In contrast to the abundance of data concerning the MVM transporter, the mechanisms for taurine transport across the BM remain to be established.
We speculated that the low plasma concentration of taurine often found in IUGR fetuses(20,21) is caused by a reduced transport capacity in the placenta. To test this hypothesis, we isolated microvillous and basal syncytiotrophoblast plasma membranes from normal and IUGR placentas and studied the transport of [3H]taurine using rapid filtration techniques.
METHODS
Patients. Patients were delivered at Sahlgrenska University Hospital, and collection of placental tissue was approved by the Committee for Research Ethics at Göteborg University (no. 144-94). Estimated gestational age was determined from last menstrual period and confirmed by ultrasound at 16-18 wk of gestation. Control placentas were obtained from uncomplicated pregnancies, delivered vaginally (n = 3) or by cesarean section (n = 10) due to maternal indication. IUGR was defined as a birth weight >2 SD lower than the mean birth weight for gestational age(22). In the IUGR group only pregnancies with no other major complication (such as preeclampsia or diabetes) were included. One patient in the IUGR group and two patients in the control group were given corticosteroids to promote fetal lung maturation. Selected clinical data for control and IUGR groups are given in Table 1. Fetal weight was 44% lower in the IUGR group, and placental weight was reduced by 48%. In Table 2 clinical data for individual IUGR placentas are presented. IUGR babies were delivered vaginally (n = 2) or by cesarean section (n = 9) due to signs of fetal distress. In 9/11 cases, abnormal Doppler blood flow patterns were registered in the umbilical artery during days or weeks before delivery. Other indications of fetal compromise in the IUGR group included oligohydramnios (four cases) and delivery by acute cesarean section (four cases) due to signs of fetal distress (decreased cardiotocography variability and tachycardia). These data suggest that the IUGR group represents fetuses subjected to true growth restriction rather than genetically or constitutionally small babies.
Preparation of the vesicles. Placentas were placed on ice immediately after delivery, and vesicle preparation was started within 1 h. MVMs and BMs were isolated and purified according to the protocol of Illsley and co-workers(23–25). In brief, after removal of the chorionic plate, amnion, and decidua, the placenta was cut into small pieces and rinsed with physiologic saline (pH 7.4 at 4°C). Using a Polytron, the tissue was homogenized in buffer D (250 mM sucrose, protease inhibitors, 10 mM HEPES-Tris, pH 7.4 at 4°C). Homogenate was centrifuged at low speed (10 000 × g, for 15 min) and the supernatant was subsequently spun at high speed (125 000 ×g, 4°C, for 30 min) to pellet a crude membrane fraction. After resuspension in buffer D, membranes were precipitated using MgCl2. MVM, the supernatant of the Mg2+ precipitation step, was centrifuged(30 min, 4°C at 125 000 × g). BM, contained in the pellet of the Mg2+ precipitation step, was purified using sucrose gradient centrifugation. Finally BM and MVM were centrifuged (30 min at 4°C, 125 000 × g) and resuspended in an appropriate volume of buffer D to give a final protein concentration of 5-10 mg × mL-1. Vesicles were snap frozen in liquid nitrogen and stored at-80°C until use in transport measurements. In selected experiments vesicles were used in uptake experiments without prior freezing.
Transport of taurine. Standard rapid filtration techniques were used to study uptake of taurine. Vesicles were loaded by incubation in loading buffer (10 mM HEPES-Tris, 300 mM mannitol, pH 7.4) overnight at 4°C. The vesicles were centrifuged (20 min, 4°C, at 504 000 ×g), resuspended in the appropriate volume of loading buffer, and placed on ice. Immediately before transport experiments, vesicles were allowed to equilibrate to room temperature. Taurine uptake was initiated by the addition of 20 µL of vesicle suspension (protein concentration 4-8 mg × mL-1) to 40 µL of incubation buffer. Buffer A(final concentration after mixing: 10 mM HEPES, 100 mM NaCl, 0.5 µM[3H]taurine, pH 7.4, at 22°C) was used as incubation buffer to measure total taurine uptake. To estimate sodium-independent taurine transport, vesicles were incubated in buffer B (final concentration after mixing: 10 mM HEPES, 100 mM KCl, 0.5 µM [3H]taurine, pH 7.4, at 22°C). Taurine uptake was terminated after 10 s to 1 h by the addition of 2 mL of ice-cold PBS. The vesicle suspension was promptly filtered on 0.45-µm pore size filters (Millipore Corp., Bedford, MA) presoaked with buffer (10 mM HEPES, 100 mM taurine, 200 mM mannitol, pH 7.4, at 22°C). After thorough rinsing (three times with 2 mL of ice-cold PBS), filters were removed, dissolved in 2 mL of scintillation fluid (Quickszint 361, Zinsser Analytic), and counted.
Assays. The protein concentration of samples was determined by Bradford assay(26). MVM and BM enrichments were assessed using standard activity assays for adenylate cyclase(27) and alkaline phosphatase(28). The production of cAMP by adenylate cyclase was measured by RIA (New England Nuclear, Boston, MA).
Data analysis. All experiments were carried out in triplicate. Sodium-dependent taurine uptake was calculated by subtracting uptakes in the presence of KCl from transport in the presence of NaCl. Differences of uptake of [3H]taurine were compared using an unpaired t test, and significance was defined at the p < 0.05 level. Time course data were analyzed by linear regression, and differences in slopes were evaluated statistically by an F test(29). Data are given as mean ± SEM.
Materials. [3H]Taurine (21 Ci/mmol) was obtained from Amersham International, England. All other chemical products were purchased from Sigma-Aldrich Sweden AB.
RESULTS
Measurement of the activity of relevant enzyme markers is a well established technique for the evaluation of the degree of purification of a membrane fraction. In general, the ratio of enzyme activity in the isolated membrane over that of placental homogenate constitutes the enrichment. In the present study, alkaline phosphatase was used as a MVM marker and forskolin-stimulated adenylate cyclase activity as a BM marker(23). Enrichment of alkaline phosphatase activity in MVM was 15.7 ± 3.1 (n = 10) in the control group and 19.4± 5.8 (n = 8) in the IUGR samples. Enrichment of adenylate cyclase activity in BM was 13.2 ± 3.1 (control, n = 5) and 11.7 ± 3.2 (IUGR, n = 7). Enrichments of marker enzymes in IUGR vesicles were not statistically different from that of controls.
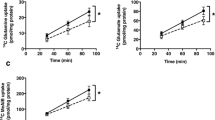
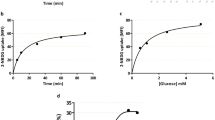
The presence of a Na+-dependent transport system for taurine in MVM was confirmed by measuring the time-dependent uptake of[3H]taurine in the presence and absence of Na+(Fig. 1). A Na+-dependent taurine uptake could also be demonstrated in BM (Fig. 2). Sodium-dependent uptake of taurine was linear from 0 to 60 s in both MVM (r = 0.922, p < 0.05) and BM (r = 0.787, p< 0.05) (Fig. 3). In subsequent studies comparing taurine uptakes in control and IUGR vesicles, 30-s (MVM) and 60-s (BM) values were taken to approximate initial rate kinetics. To evaluate the effect of vesicle freezing on taurine transport the time course of taurine uptake in freshly prepared and snap frozen vesicles was compared. Because uptakes in frozen vesicles were not significantly different (Fig. 4), frozen vesicles were used in all subsequent studies.
Time course of sodium-dependent uptake of [3H]taurine in freshly prepared (n = 3) and frozen(n = 4) MVM (A) and BM vesicles (B). Slopes(pmol × mg protein-1 × s-1) of linear regression lines were compared statistically using an F test and were found to be similar in fresh and frozen vesicles both in MVM (kfresh = 0.077, r = 0.83, and kfrozen = 0.065, r = 0.71) and BM(kfresh = 0.0054 r = 0.74 and kfrozen = 0.0073, r = 0.79).
Taurine uptake was measured in membrane vesicles obtained from control and IUGR placentas with a gestational age ranging from 32 to 41 wk. Within this range of gestational ages, taurine uptake rates were independent of length of pregnancy in both groups (data not shown). Thus, data from preterm and term placentas were pooled. In control MVM, Na+-independent taurine represented less than 3% of total taurine uptake (Table 3). In control BM, Na+-dependent taurine was only 6% of that in MVM, whereas Na+-independent taurine was similar to the MVM value(Table 3).
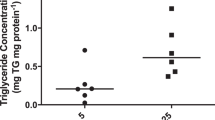
Taurine uptake data for the individual IUGR placentas are presented in Table 4. In MVM isolated from IUGR placentas, Na+-dependent taurine uptake (2.26 ± 0.25 pmol × mg protein-1 × 30 s-1) was 34% lower (p < 0.05) compared with control values (Fig. 5). MVM Na+-independent taurine transport in IUGR (0.11 ± 0.026 pmol× mg protein-1 × 30 s-1) was not different compared with controls (Table 3). In BM, Na+-dependent taurine uptake was unaffected in IUGR (0.37 ± 0.05 pmol × mg protein-1 × 60 s-1) compared with control values (Table 3). In contrast, the Na+-independent component of taurine uptake in BM was reduced by 33%(p < 0.05) in IUGR (0.075 ± 0.010 pmol × mg protein-1 × 60 s-1) (Fig. 6).
DISCUSSION
Taurine is the most abundant free amino acid present in mammalian tissues, intracellular concentrations commonly exceeding 10 mM(30). Taurine is an end product of sulfur amino acid metabolism and is not incorporated into proteins due to the β-position of the amino group. Apart from a well documented role in bile acid conjugation, the physiologic function of this amino acid remains elusive. There is, however, a mounting body of evidence that taurine plays an important role in cellular osmoregulation(31), neuromodulation(7), membrane stabilization(32), and defense against oxygen free radicals(33).
Normal fetal and neonatal development is critically dependent on the availability of taurine. In animal experiments, including primate models, taurine deficiency during pregnancy and lactation is associated with growth failure, abnormal cerebellar development, neurologic deficits, retinal degeneration, and cardiac damage(11). In human infants on total parenteral nutrition lacking taurine, low plasma concentrations of taurine have been associated to visual impairments(11). These findings, together with studies in the monkey, suggest that taurine deficiency may have detrimental effects also in human development, supporting the rationale of taurine supplementation in commercial formulas and in parenteral nutrition regimens for infants(11,34). Due to low or absent activity of cysteine sulfinic acid decarboxylase, the rate-limiting step in the taurine synthetic pathway, in human fetal tissues(12,13) placental transport of taurine is vital to the growth and development of the fetus. Consequently, to better understand the mechanisms underlying the development of altered growth pattern in IUGR it is important to identify changes in placental taurine transport associated with this pregnancy complication.
Studies of the taurine transport mechanisms across MVM in human placenta demonstrate the presence of a single, high affinity, Na+- and Cl--dependent transport system highly selective for β-amino acids(15–18). In the present study, the addition of extravesicular Na+ stimulated taurine uptake in MVM vesicles 35-fold, confirming the Na+ dependence of taurine transport in the maternal facing plasma membrane of the syncytiotrophoblast. Taurine transport mechanisms in BM have, to our knowledge, not been studied. Approximately 75% of total taurine uptake in BM could be accounted for by a Na+-dependent transporter. Direct comparisons of transport activities between two different membrane preparations are subjected to a variety of potential sources of error. However, previous studies using the current membrane isolation procedure suggest that MVM and BM are quite similar in characteristics that could affect such a comparison. For example, the fraction of sealed vesicles appear to be close to 100% in both membranes(25), the majority of vesicles have been shown to be oriented right-side-out in BM and MVM(23) and the phospholipid/protein ratio is only 30% higher in BM(35). Therefore, we feel that measured transport activities can, with some caution, be compared between the two plasma membrane fractions.
It is interesting to note that the activity of the Na+-dependent taurine transporter in BM was only 6% that of the corresponding activity in MVM. In addition, Na+-independent transport rates were similar in MVM and BM. We suggest that these findings provide the basis for net taurine flux from the mother to the fetus. In MVM, the Na+/taurine cotransporter, energized by the inwardly directed Na+ gradient, will efficiently accumulate taurine inside the syncytiotrophoblast cell, and backflux into the maternal circulation will be small due to the minute contribution of Na+-independent pathways to overall fluxes. In BM, taurine transport in the fetal direction mediated by Na+-independent pathways is strongly favored by the steep outwardly directed taurine gradient. Uphill transport from the fetal circulation into the syncytiotrophoblast cell is limited due to the relatively low activity of Na+-dependent pathways in BM. The present study was not designed to investigate taurine transport mechanisms in BM in detail. It remains to be established whether the Na+-dependent taurine transport activity in BM has the same kinetic characteristics as the MVM transporter. In addition, the nature of the Na+-independent pathway for taurine transport is unknown at present.
IUGR was associated with two distinct alterations in the placental transport system for taurine. First, Na+-dependent taurine uptake was reduced by 34% in MVM isolated from IUGR placentas. Second, the transport rate of Na+-independent pathways in BM were 33% lower in IUGR. These two changes will work in concert to decrease materno-fetal taurine transport in IUGR, and we suggest that the low plasma concentrations of taurine often found in IUGR fetuses(20,21) are caused by a reduced activity of placental taurine transporters.
The signals eliciting alterations in the fetal growth pattern in response to a compromised maternal supply line have not been elucidated in detail. IUGR fetuses are sometimes hypoinsulinemic(36) due to hypoglycemia in utero(37,38), and the low insulin levels will slow the growth rate in insulin-sensitive tissues such as skeletal muscle and liver, whereas brain growth is relatively spared. The low plasma concentrations of IGF-I(39) of IUGR fetuses as well as the redistribution of cardiac output to prioritized organs(40) will have similar effects. Taurine deficiency in pregnancy results in IUGR in cats(10) and rats(41), suggesting that taurine availability might regulate fetal growth directly. Thus, it is possible that the reduced placental transport capacity for taurine in IUGR represent another key signal for the altered fetal growth pattern in this pregnancy complication.
Abbreviations
- IUGR:
-
intrauterine growth restriction
- MVM:
-
microvillous membrane
- BM:
-
basal membrane
References
Fanaroff AA, Wright LL, Stevenson DK, Shankaran S, Donovan EF, Ehrenkranz RA, Younes N, Korones SB, Stoll BJ, Tyson JE, Bauer CR, Oh W, Lemons JA, Papile LA, Vertex J 1995 Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991 through December 1992. Am J Obstet Gynecol 173: 1423–1431
Blair E, Stanley F 1990 Intrauterine growth and spastic cerebral palsy. I. Association with birth weight for gestational age. Am J Obstet Gynecol 162: 229–237
Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS 1993 Fetal nutrition and cardiovascular disease in adult life. Lancet 341: 938–941
Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall C, Osmond C, Winter PD 1991 Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303: 1019–1022
Dicke JM, Henderson GI 1988 Placental amino acid uptake in normal and complicated pregnancies. Am J Med Sci 295: 223–227
Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RDH, Sibley CP 1993 Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34: 661–665
Kuriyama K 1980 Taurine as a neuromodulator. Fed Proc 39: 2680–2684
Shennan DB, McNeillie SA, Curran DE 1993 Stimulation of taurine efflux from human placental tissue by a hyposmotic challenge. Exp Physiol 78: 843–846
Hayes KC, Carey RE, Schmidt SV 1975 Retinal degeneration associated with taurine deficiency in the cat. Science 188: 949–951
Sturman JA, Gargano AD, Messing JM, Imaki H 1986 Feline maternal taurine deficiency: effect on mother and offspring. J Nutr 116: 655–667
Sturman JA 1988 Taurine in development. J Nutr 118: 1169–1176
Hayes KC, Sturman JA 1981 Taurine in metabolism. Annu Rev Metabol 1: 401–425
Gaull G, Sturman JA, Räihä NCR 1972 Development of mammalian sulphur metabolism: absence of cystathionase in human fetal tissue. Pediatr Res 6: 538–547
Philipps AF, Holzman IR, Teng C, Battaglia FC 1978 Tissue concentrations of free amino acids in term human placentas. Am J Obstet Gynecol 131: 881–887
Miyamoto Y, Balkovetz DF, Leibach FH, Mahesh VB, Ganapathy V 1988 Na+Cl- gradient-driven, high affinity, uphill transport of taurine in human placental brush-border membrane vesicles. FEBS Lett 231: 263–267
Karl PI, Fischer SE 1990 Taurine transport by microvillous membrane vesicles and in the perfused cotyledon of the human placenta. Am J Physiol 258:C443–C451
Moyer MS, Insler N, Dumaswala R 1992 The role of chloride in taurine transport across human placental brush-border membrane. Biochim Biophys Acta 1109: 74–80
Kulanthaivel P, Leibach FH, Mahesh VB, Ganapathy V 1989 Tyrosine residues are essential for the activity of the human placental taurine transporter. Biochim Biophys Acta 985: 139–146
Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V 1994 Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem J 300: 893–300
Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC 1990 Umbilical amino acid concentrations in normal and growth retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162: 253–261
Economides DL, Nicolaides KH, Gahl WA, Bernardini I, Evans MI 1989 Plasma amino acids in appropriate- and small-for-gestational-age fetus. Am J Obstet Gynecol 161: 1219–1227
Marsál K, Persson P-H, Larsen T, Lilja H, Selbing A, Sultan B 1996 Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 85: 843–848
Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM 1990 Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta 1029: 218–226
Jansson T, Powell TL, Illsley NP 1993 Non-electrolyte solute permeabilities of human placental microvillous and basal membranes. J Physiol 468: 261–274
Jansson T, Illsley NP 1993 Osmotic water permeabilities of human placental microvillous and basal membranes. J Membr Biol 132: 147–155
Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Schultz G, Jakobs KH 1984 Adenylate cyclase. In: Bergmeyer H (ed) Methods of Enzymatic Analysis. Verlag Chemie, Weinham, Germany, PP 369–378
Bowers G, McComb R 1966 A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin Chem 12: 70–89
Snedecor GW, Cochran WG 1980 Statistical Methods. The Iowa State University Press, Ames, IA, PP 385–388
Sturman JA, Hayes KC 1980 The biology of taurine in nutrition and development. Adv Nutr Res, PP 231–299
Kirk K 1997 Swelling-activated organic osmolyte channels. J Membr Biol 158: 1–16
Hamaguchi T, Azuma J, Schaffer S 1991 Interaction of taurine with methionine: inhibition of myocardial phospholipid methyltransferase. J Cardiovasc Pharmacol 18: 224–230
Halliwell B 1987 Oxidants and human disease: some new concepts. FASEB J 1: 358–364
Ghisolfi J 1987 Taurine and the premature. Biol Neonate 52( suppl 1): 78–86
Jansson T, Wennergren M, Illsley NP 1993 Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 77: 1554–1562
Economides DL, Proudler A, Nicolaides KH 1989 Plasma insulin in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 160: 1091–1094
Economides DL, Nicolaides KH 1989 Blood glucose and oxygen tension levels in small-for-gestational-age fetuses. Am J Obstet Gynecol 160: 385–389
Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G 1996 The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet Gynecol 87: 937–942
Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M 1991 Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res 29: 219–225
Wladimiroff JW, Tonge HM, Stewart PA 1986 Doppler ultrasound assessment of cerebral blood flow in the human fetus. Br J Obstet Gynaecol 93: 471–475
Ejiri K, Akahori S, Kudo K, Sekiba K, Ubuka T 1987 Effect of guanidinoethyl sulfonate on taurine concentrations and fetal growth in pregnant rats. Biol Neonate 51: 234–240
Author information
Authors and Affiliations
Additional information
Supported by Swedish Medical Research Council (10838, 11834 and 02591), the Axel and Margaret Ax:son Johnson Foundation, the Emil and Vera Cornell Foundation, the Bank of Sweden Tercentenary Foundation, Frimurare-Barnhus-direktionen, the Swedish Society for Medical Research, theÅhlens Foundation, the Lars Hierta Foundation, the Samariten Foundation, the Sven Jerring Foundation, the General Maternity Hospital Foundation, the Magnus Bergvall Foundation, the Craaford Foundation, and the Willhelm and Martina Lundgrens Foundation. S.N. was supported by the Wera Ekström Foundation for Pediatric Research.
Rights and permissions
About this article
Cite this article
Norberg, S., Powell, T. & Jansson, T. Intrauterine Growth Restriction Is Associated with a Reduced Activity of Placental Taurine Transporters. Pediatr Res 44, 233–238 (1998). https://doi.org/10.1203/00006450-199808000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199808000-00016
This article is cited by
-
CHOP upregulation and dysregulation of the mature form of the SNAT2 amino acid transporter in the placentas from small for gestational age newborns
Cell Communication and Signaling (2023)
-
Sexually dimorphic patterns in maternal circulating microRNAs in pregnancies complicated by fetal growth restriction
Biology of Sex Differences (2021)
-
Fetal sex modulates placental microRNA expression, potential microRNA-mRNA interactions, and levels of amino acid transporter expression and substrates: INFAT study subpopulation analysis of n-3 LCPUFA intervention during pregnancy and associations with offspring body composition
BMC Molecular and Cell Biology (2021)
-
Birth of a pathway for sulfur metabolism in early amniote evolution
Nature Ecology & Evolution (2020)
-
N-Acetylcysteine protects against intrauterine growth retardation-induced intestinal injury via restoring redox status and mitochondrial function in neonatal piglets
European Journal of Nutrition (2019)