Abstract
α-Tocopherol and fish oil have been reported to modulate the progression of IgA nephropathy in animals and humans. Because fish oil has been reported to exacerbate renal disease in subtotal nephrectomized rats, we investigated the effects of fish oil, with and without α-tocopherol, on the course of IgA nephropathy. Experimental IgA nephropathy was induced in male Sprague-Dawley rats, weighing 170-200 g, by oral and i.v. immunization with bovine γ-globulin for 8 wk. IgA nephropathy was evidenced by hematuria, proteinuria, and IgA deposition in the mesangium. Standard rodent chow, containing 30 IU of α-tocopherol/kg of diet, was given to the control and IgA nephropathy rats. Fish oil (20% wt/wt), stripped ofα-tocopherol preservative, was given to control and a second group of IgA nephropathy rats. Alternatively, corn oil or fish oil was supplemented with α-tocopherol at 100 IU/kg of diet and given to the third and fourth groups of IgA nephropathy rats. All animals were killed at 8 wk. Urinary protein excretion, plasma and kidney α-tocopherol concentrations, as well as glomerular planar area, and kidney transforming growth factor-β1 mRNA were analyzed. As determined by reductions in proteinuria, glomerular planar area, and TGF-β1 mRNA, fish oil with α-tocopherol ameliorated the renal injury induced by bovineγ-globulin, whereas fish oil without α-tocopherol did not. Our findings support the importance of α-tocopherol, more so than fish oil, in mitigating the injury and promoting repair in experimental IgA nephropathy.
Similar content being viewed by others
Main
IgA nephropathy, previously known as Berger's disease(1) and thought to be a benign codition of macroscopic hematuria associated with upper respiratory infections, is now regarded as a major cause of end-stage renal disease. It accounts for up to 30% of patients receiving dialysis and kidney transplantation(2–4). Various therapeutic maneuvers have been promoted, including gluten-free diets, tonsillectomies, dipyridamole, phenytoin, steroids, cyclosporine, and angiotensin-converting enzyme inhibitors, but none have been proven to be effective(4–6). Recently, oral administration of fish oil over 2 y in IgA nephropathy patients with proteinuria in excess of 1 g/d significantly retarded the rate of deterioration of renal function(7). The active ingredient of fish oil, ω-3 polyunsaturated fatty acid, was thought to be responsible for reducing circulating immune complexes and lowering triglycerides and platelet aggregation.
Although the natural history of IgA nephropathy is well known(2–6), its pathogenesis is still unclear(8). Recently, several lines of evidence supported the concept that the pathogenesis of IgA nephropathy is related to free radical injury(9–11) and thusα-tocopherol, acting synergistically as an antioxidant preservative of fish oil, may account for part or all of the beneficial effects. Therefore, we designed this experiment to investigate the effects of fish oil, stripped ofα-tocopherol preservative, on glomerular proliferation and fibrogenic cytokine expression in experimental IgA nephropathy, and to compare these parameters with fish oil and α-tocopherol and with α-tocopherol supplementation alone.
METHODS
This experimental protocol has been approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University's Medical College of Virginia. Male Sprague-Dawley rats, weighing 170-200 g, were purchased from Harlan Laboratories (Indianapolis, IN). They were maintained in standard cages at 25 °C with a 12 h light-dark cycle. The animals were provided standard rodent chow (Purina Mills, Inc., Richmond, IN) and water to drink ad libitum for 1 wk before the initiation of the studies. Four to six weight-matched animals were assigned to each group. Rats receiving standard rodent chow and water ad libitum served as the controls. Control animals received no BGG throughout the experiment; they were given 0.2 mL of normal saline i.v. for three consecutive days at the end of the experiment.
IgA nephropathy model. Rats were orally immunized with 0.1% BGG(G7516 from Sigma Chemical Co., St. Louis, MO) in a 6 mM HCl solution in the drinking water(12). At the end of the 8 wk of oral immunization, each rat also received 1 mg (0.2 mL) per day of BGG i.v. for three consecutive days(12). Rats were then killed by injection of pentobarbital (50 mg/kg body weight) and exsanguination, as described in our previous studies(11, 13).
Standard chow and α-tocopherol diets. Control and untreated IgA nephropathy animals received a standard rodent chow, which contained 30 IU of α-tocopherol/kg of chow. Other groups of control and IgA nephropathy rats were fed a specially prepared diet of fish oil (detailed below) without α-tocopherol or supplemented with 100 IU ofα-tocopherol/kg of chow(10) or corn oil supplemented with 100 IU of α-tocopherol/kg of chow. The fish oil used in these studies, as in almost all other such studies, was supplied by the Fish Oil Test Materials Program of the National Marine Fisheries Service, Southeast Fisheries Science Center, Charleston, SC. The deodorized menhaden oil contains approximately 30% n-3 fatty acids as triglycerides: 14% eicosanoic acid, 8% docosahexanoic acid, and 8% other n-3 fatty acids. The fish oil was deodorized and stripped of α-tocopherol under vacuum; resultant concentrations of α-tocopherol were 20-30 μg/g of fish oil. The menhaden oil was preserved by the addition of 0.01% TBHQ before shipment to our laboratory, rather than with α-tocopherol, which is customarily used. The detailed analysis, which was performed after vacuum stripping, is provided in Table 1. Periodic testing of the chow indicated that there was no bacterial or fungal contamination or increased rancidity. All control animals were also provided a 6 mM HCl solution of drinking water.
Fish oil diet without α-tocopherol. A defined diet, TD 96133, deficient in α-tocopherol and designed for reconstitution with 21.2% fat, was obtained from Harlan Teklad Laboratories (Madison, WI). This basal food mix was supplemented as follows: 20% menhaden oil, withoutα-tocopherol (preserved with TBHQ as Tenox-20A, 0.2 g/kg); 1% corn oil, stripped of α-tocopherol (preserved with TBHQ as Tenox-20A, 0.2 g/kg).
Fish oil diet plus α-tocopherol. A defined diet, TD 96133, deficient in α-tocopherol and designed for reconstitution with 21.2% fat, obtained from Harlan Teklad Laboratories (Madison, WI), was used with the following supplements: 20% menhaden oil, without α-tocopherol(preserved with TBHQ as Tenox-20A, 0.2 g/kg); 1% corn oil containingα-tocopherol at 0.2 g/kg; and 100 IU of α-tocopherol/kg of diet. The 20% menhaden oil, plus the 1% corn oil, with 0.2 g/kg α-tocopherol, gave a fat content of 21.2%.
Corn oil diet plus α-tocopherol. The following corn oil diet was used: 21% corn oil (ICN no. 901415) containing α-tocopherol at 0.2 g/kg was supplemented with 100 IU of α-tocopherol/kg of diet to provide a 21.2% fat content, identical to the fat content of all the other diets.
Menhaden oil was generously provided by the Biomedical Test Materials Program of the National Marine Fisheries Service, Southeast Fisheries Science Center, Charleston, SC. Tenox-20A was a gift from Eastman Chemical Products, Kingsport, TN. Corn oil was purchased from ICN Pharmaceuticals, Inc. (Costa Mesa, CA); α-tocopherol was obtained from Sigma Chemical Co.
General comments on the different diets. Administration of BGG,i.e., development of IgA nephropathy, was concurrent to provision of the appropriate special diet. Specifically, the fish oil/α-tocopherol/corn oil/control diets were initiated the day after the arrival of the animals at this facility. BGG was provided beginning 2 d after that. The 3-d consecutive i.v. injections of BGG were begun 8 wk (half the animals) or 8 wk and 1 d (remaining rats) after oral immunization with BGG was initiated.
Rats were not pair-fed, as this was deemed unnecessary in view of our previous experience(11). Food consumption did not differ greatly between the control animals and their corresponding experimental groups. Although there were differences between the chow-fed animals and those provided the high fat diets in total weight of food consumed, caloric intakes did not differ.
Urine and blood collection. After completion of the 8 wk of oral immunization and the 3-d i.v. immunization period, animals were placed in individual metabolic cages (Nalge, Rochester, NY), and 24-h urine samples were collected for determination of protein concentrations. Rats were given free access to water, but not food, while they were in the metabolic cages.
Urine protein concentrations were measured using the Coomassie Blue binding reagent (Bio-Rad, Richmond, CA). By cardiac puncture exsanguination, a fasting blood sample was obtained at the end of the urine collection for measurement of α-tocopherol.
Histopathology examination. The kidneys were immersionfixed in 10% formalin solution and stained with hematoxylineosin and periodic acid-Schiff reagents.
Glomerular planar area was determined by surveying 40-50 glomeruli in each kidney specimen with a computerized digitizing pad (Micro-plan II, Laboratory Computer Systems, Inc., Natick, MA). Glomeruli throughout the cortex with clear delineation of the hilar region were included in this analysis(11).
Immunofluorescence examination of the renal tissue(11) was performed by staining snap frozen sections with FITC-labeled anti-rat IgA antibodies (1:100 dilution; Bethyl Laboratories, Montgomery, TX). The nephrologist (H.T.) who examined the specimens was unaware of the group assignments of the individual animals.
mRNA isolation and Northern blotting. Total RNA was isolated from renal cortical tissue using the Trizol reagent (Life Technologies, Inc., Gaithersburg, MD) and quantitated spectrophotometrically at 260 nm. The RNA was then applied to a denaturing 0.8% agarose gel, and the integrity of the RNA was assessed by ethidium bromide staining of the 28 and 18 S ribosomal RNA bands. The abundance of TGF-β1 mRNA was determined using an RNase protection assay(14) with a rat TGF-β1 probe (catalog no. 63197, ATCC, Rockville, MD). Cyclophilin was used as an internal standard.
α-Tocopherol analysis. Serum α-tocopherol analyses were performed using reversed-phase HPLC methodology(15). The investigator (E.P.N.) who performed these analyses was blinded to their identification. Briefly, 100-μL aliquots of serum were mixed with 2 volumes of a 1% (wt/vol) solution of ethanolic L-ascorbic acid that also contained an internal standard (retinyl proprionate). Samples were mixed by vortex for 30 s, and 2 mL of n- hexane, containing 1% butylated hydroxytoluene, added. Samples were shaken for 10 min while protected from light. After centrifugation (5 min, 4 °C, 250 × g), the hexane layer was removed, and an additional 2-mL solution of 1% butylated hydroxytoluene in hexane was added. Samples were again shaken and centrifuged as described, and both hexane layers were combined. The entire hexane extract then was evaporated to dryness under N2. The sample residue containing α-tocopherol and internal standard was reconstituted at 200 μL of mobile phase (50:50, acetonitrile:ethanol that contained 0.1% diethylamine). 20 μL of sample were injected onto a reversed-phase C-18, 5-μm, 4.6 × 150-mm column.α-Tocopherol in serum was detected at 292 nm and quantitated using standard curve data obtained in the same HPLC run.
Kidney samples were assayed for α-tocopherol using the same methodology as for serum. However, before extraction into n-hexane, 20-μg samples of kidney were homogenized in ethanol and saponified for 20 min at 78 °C(16).
Statistical analysis. The differences between groups were examined by using t test and ANOVA where appropriate with Duncan used as a post hoc test(11, 14). The experimental findings were considered statistically significant if p< 0.05.
RESULTS
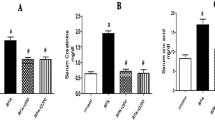
Urinary protein concentrations. Figure 1 shows that urinary protein excretion was significantly elevated in the untreated and fish oil-treated IgA nephropathy rats compared with the untreated and fish oil-treated control animals. This elevated proteinuria returned to values intermediate between the elevated and control values in the IgA nephropathy rats treated with α-tocopherol in conjunction with fish oil or corn oil.
Urinary protein excretion at end of 8 wk of the experiment. Values presented in milligrams/d (mean ± SEM). Control animals in open bars and IgA nephropathy animals in stippled bars. Significant elevation of proteinuria occurred in IgA nephropathy animals given standard chow and also those given fish oil without vitamin E supplementation. With α-tocopherol supplementation in fish oil or corn oil, very significant reductions were found in the urinary protein excretion. Different superscripts denote significant differences between groups not sharing the same superscript. Identical superscripts denote no significant difference.
Plasma and kidney α-tocopherol concentrations.Figures 2 and 3 show plasma and kidneyα-tocopherol concentrations, respectively. In the control animals on regular chow (Fig. 2), the mean ± SEM of plasmaα-tocopherol concentrations (808 ± 20) μg/dL were no different from those in IgA nephropathy rats on regular chow (835 ± 80 μg/dL), but both were significantly higher than animals on the stripped fish oil diets. A significant rise in plasma α-tocopherol to 598 ± 60 and 1200 ± 110 μg/dL occurred in IgA nephropathy rats on fish oil or corn oil supplemented with α-tocopherol, respectively.Figure 3 shows the same pattern of kidney concentrations of α-tocopherol with undetectable levels in the α-tocopherol-free fish oil groups and markedly elevated values in theα-tocopherol-supplemented groups.
Plasma α-tocopherol. Control animals in open bars and IgA nephropathy animals in stippled bars. Values presented as mean and SEM. In control animals as well as in IgA nephropathy animals given fish oil without α-tocopherol supplementation, the plasma α-tocopherol concentration was close to zero.α-Tocopherol supplemented animals showed significant elevation. Different superscripts denote significant difference between groups not sharing the same superscript. Identical superscripts denote no statistical difference.
Kidney α-tocopherol. Values presented in milligrams/g of tissue, means ± SEM. Control animals in open bars and IgA nephropathy animals in stippled bars. In both control as well as IgA nephropathy animals given fish oil withoutα-tocopherol supplementation, kidney α-tocopherol concentration was undetectable. Supplementation with α-tocopherol resulted in significant rise in kidney content of α-tocopherol. Different superscripts denote significant differences between groups not sharing the same superscript. Identical superscripts denote no statistical difference.
Immunopathology and glomerular planar area. The glomerular mesangial IgA deposition varied from trace to 2+ in all BGG-vaccinated rats and was absent in the control animals. Dietary supplementation with fish oil, corn oil, or α-tocopherol did not change the intensity of IgA deposition in the mesangial region.
Figure 4 presents the glomerular planar area in the control animals versus the IgA nephropathy animals. The IgA nephropathy rats on regular chow showed enlargement of glomerular planar area(×10-3 mm2) to 147 ± 9 (mean ± SEM), which was significantly higher than the control animals receiving chow (122 ± 5) or fish oil without α-tocopherol (114 ± 4). The administration of fish oil alone (i.e. fish oil without α-tocopherol as antioxidant preservative) resulted in no significant reduction of the glomerular planar area (138 ± 3) compared with the IgA nephropathy rats(147 ± 9) receiving chow. However, in IgA nephropathy rats on diets of fish oil or corn oil supplemented with α-tocopherol, the glomerular planar areas were reduced to 126 ± 2 and 124 ± 4, respectively. Both were significantly lower than the IgA nephropathy rats on fish oil alone(138 ± 3) or chow (147 ± 9). Indeed,α-tocopherol-supplemented IgA nephropathy rats showed glomerular planar areas (126 ± 2; 124 ± 4) no different from those of the control animals (122 ± 5).
Glomerular planar area as an index of glomerular mesangial proliferation. Control animals in open bars and IgA nephropathy animals in stippled bars. Values shown in mean ± SEM. Different superscripts denote statistically significant differences between groups not sharing the same superscripts. Identical superscripts indicate no significant differences. IgA nephropathy animals given standard animal chow showed significantly elevated glomerular planar area. The use of fish oil in such animals showed no significant change. When IgA nephropathy animals were given fish oil supplemented with α-tocopherol or corn oil supplemented with α-tocopherol, significant inhibition of proliferation was demonstrated, with values no different from those of control animals.
Kidney TGF-β1 mRNA. The kidney TGF-β1 mRNA pattern in the control and IgA nephropathy animals was very similar to that of the glomerular planar area (Fig. 5). The lowest kidney TGF-β1 expression was associated with control rats provided regular chow. Control rats provided the fish oil diet exhibited higher concentrations of renal TGF-β1 expression, albeit not as high as the IgA nephropathy group given regular chow. Kidney TGF-β1 expression was diminished in the IgA nephropathy animals which ingested the fish oil-containing diet. A large and significant decrement in TGF-β1 expression occurred in the IgA nephropathy animals given α-tocopherol.
Kidney TGF-β1 mRNA, in arbitrary densitometry units (ADU). Data presented as mean and SEM, with control animals in open bars and IgA nephropathy animals in stippled bars. Different superscripts denote significant differences between groups not sharing the same superscript. Identical superscripts denote no significant statistical difference. Kidney TGF-β1 mRNA was significantly elevated in IgA nephropathy animals given standard chow. This came down to control values in IgA nephropathy animals given fish oil withα-tocopherol or corn oil with α-tocopherol.
DISCUSSION
Recently, Donadio et al.(7) reported in a double-blinded study, in a large consortium of patients with IgA nephropathy associated with heavy proteinuria (in excess of 1 g/d), that fish oil supplementation reduced the proteinuria and slowed the rate of increase in serum creatinine. However, several other studies(17–19) showed lack of such a renal protective effect of fish oil. These later studies used fish oil in a smaller number of patients(17–19) and only one was double blinded(19). Because the use of fish oil offered such promise in this disease, which heretofore lacked any proven treatment, we sought to delineate the effects of fish oil in an IgA nephropathy model in the rat(12), a chronic BGG model that we have used in previous studies(11, 13). In another progressive renal disease model, Scharschmidt et al.(20) reported that fish oil was detrimental to renal function in the remnant kidney uremic rats. Tateno et al.(21), using B10.Br mice in a model of serum sickness nephritis, found that fish oil supplementation exacerbated proteinuria and the severity of renal histologic changes. In a study by Clarke et al.(22), fish oil protected the remnant kidney if a specific antioxidant, α-tocopherol, was used in conjunction with fish oil. It was uncertain whether such results applied to other chronic renal disease models, and especially to the IgA nephropathy model. Therefore, we designed this experiment to distinguish the effects of fish oil from those ofα-tocopherol, by using specially designed diets in which theα-tocopherol was stripped from the fish oil and compared with diets in which α-tocopherol was added to corn oil or added to fish oil at a therapeutic dose. This dose has been previously shown to be protective of renal functions in the IgA nephropathy model(11).
TGF-β1 was markedly elevated in IgA nephropathy rats given standard chow (Fig. 5), as already shown in one of our previous studies(11). Our present study showed for the first time that the use of fish oil or corn oil with α-tocopherol significantly reduced such fibrogenic cytokine expression in the kidney. In the two groups given fish oil unsupplemented with α-tocopherol, the kidney TGF-β1 mRNA was elevated (Fig. 5). In IgA nephropathy animals, supplementation with α-tocopherol resulted in significant reductions of this fibrogenic cytokine to control values. Such data support the contention that α-tocopherol, and not fish oil, plays an important protective role in alleviating the glomerular injury in the induction of IgA nephropathy.
This conclusion is in accord with the data in other models of progressive renal diseases. Clark et al.(22) demonstrated that fish oil's prevention of glomerulosclerosis in the remnant kidney may be related to supplementation with α-tocopherol. Scharschmidt et al.(20), in the subtotal nephrectomy model of chronic renal insufficiency, demonstrated that 12 wk of fish oil supplementation led to severe proteinuria and glomerulosclerosis. Thus, the potential renal protection attributable to fish oil suppression of the production of cyclooxygenase metabolite, thromboxane, may not apply in the remnant kidney model(23). Although thromboxane synthase inhibition ameliorated the progression in subtotal renal ablation(24), fish oil-enriched diet may not be equivalent to thromboxane synthase inhibitor therapy.
The mechanisms of how fish oil ameliorated the rate of progression in IgA nephropathy patients were not elucidated in the double-blinded clinical trial of Donadio et al.(7). The active element in fish oil is ω-3-polyunsaturated fatty acid(2, 7). It could be reasoned that an increase in the renal content of eicosapentaenoic acid reduced arachidonic acid availability for thromboxane production and promoted series 5 leukotrienes and series 3 prostaglandins with subsequent suppression of glomerular inflammatory reaction and scarring. In contrast to the patient data of Donadio et al.(7), the elevated proteinuria in our IgA nephropathy rats was not reduced with fish oil alone (Fig. 1). When fish oil alone was used in IgA nephropathic rats, the proteinuria was higher than the IgA nephropathic rats on regular chow. The proteinuria was reduced to control values when the fish oil was supplemented with α-tocopherol. This beneficial effect of α-tocopherol was also clearly demonstrated in the corn oil plus α-tocopherol treated IgA nephropathy rats (Fig. 1). The corn oil provided dietary fat content in the same proportion to caloric intake in the diets of all the other groups of animals. Thus, in the IgA nephropathy model, our data duplicated what Clarke et al.(22) observed in the remnant kidney model, namely that fish oil is protective if it is supplemented withα-tocopherol. The presence of α-tocopherol in fish oil supplementation in the study by Donadio et al.(7) may account in part for the renal protective effect, and may explain the lack of protection of fish oil in several other studies of patients with chronic renal failure(17–19), in which the amount ofα-tocopherol in the diet was not stated.
Although there is no linear correlation between plasma or kidneyα-tocopherol concentration and the severity of disease, the following relationships obtain. First, between the control and BGG-treated animals receiving standard lab chow, which contains 30 IU of α-tocopherol/kg of chow, plasma α-tocopherol concentrations were not very different, and there was a nonsignificant difference between their kidney α-tocopherol concentrations. Nonetheless, BGG-fed animals exhibited IgA deposits (by immunofluorescence staining), whereas their control counterparts did not. Additionally, BGG-fed rats excreted significantly more protein in their urine than did their chow-fed controls. Moreover, TGF-β1 mRNA and glomerular planar area were significantly greater in chow-fed rats provided BGG than their chow-fed controls. Thus 30 IU of α-tocopherol/kg of chow is not sufficient to prevent renal injury due to oral immunization with BGG. A possible explanation for the apparent lack of correlation is the association of α-tocopherol with lipids and lipid-containing membranes.α-Tocopherol in lipid reservoirs-quite possibly where it exerts its protective effects, complexed with lipids-would not be reflected in plasma concentrations, and would be detected in the kidney only to the extent of its association with lipid-containing membranes. Conversely, plasma concentration of α-tocopherol would not serve as an indicator of its presence in membranes or other lipid reservoirs.
The true means by which α-tocopherol confers protection may be independent of its association with lipids. It is conceivable thatα-tocopherol influences kidney metabolism indirectly or affects general detoxification processes in ways we have not yet discerned. The plasma and kidney concentrations of α-tocopherol do not readily suggest a mechanism for its beneficial effects in the retardation of experimental IgA nephropathy.
Second, in the absence of dietary α-tocopherol, i.e. in the fish oil-fed control and BGG animals, the extent of renal damage was dramatically greater in animals given BGG by all criteria used in this study. These two treatment groups were used to demonstrate that IgA nephropathy developed when animals were provided dietary fish oil in addition to BGG, which was not anticipated in light of the Mayo Clinic trial of fish oil(7). Dietary α-tocopherol clearly ameliorated proteinuria, induction of TGF-β1 mRNA, and the enlargement of glomerular planar area.
Figures 2 and 3 provide an interesting insight into the mechanism of α-tocopherol's effectiveness in ameliorating manifestations of renal disease. Comparison of α-tocopherol concentrations in animals provided the α-tocopherol-supplemented fish oil and corn oil diets reveals a great disparity between plasma concentrations without dissimilarity in kidney concentrations. These data suggest thatα-tocopherol is associated with fish oil, perhaps sequestered with lipids or incorporated into membranes with the fish oil, rendering the lipids less susceptible to oxidation(25). The lower plasma and kidney levels of α-tocopherol in fish oil-fed rats was an unanticipated result, although similar-albeit less dramatic-observations have been noted in human(26) and animal(27) studies. Although we do not fully understand these data, we hypothesize thatα-tocopherol is complexed with fish oil and remains associated with it whether it is metabolized and excreted, incorporated into membranes, or stored as lipid. Thus fish oil effectively depletes α-tocopherol from plasma and many tissues, except as a lipid component of membranes. It is difficult to attribute the beneficial effects of α-tocopherol to changes in plasma or kidney concentrations. However, following the argument set forth above, its effects may be due to its ability to prevent oxidation of membrane-bound lipids, and thus may be attributable to changes in α-tocopherol status not reflected adequately in parameters we have presented in this work. However, it should be noted that not all antioxidants mitigate renal damage. The fish oil is preserved with TBHQ as Tenox-20A, which is obviously not effective in preventing BGG-mediated impairment. An additional example is probucol, which ameliorates renal damage, whereas vitamin C does not(25). Thus there is a relationship betweenα-tocopherol administration and the amelioration of renal damage despite the lack of linear correlation between plasma or kidney concentrations and extent of injury.
Using the glomerular planar area as an index of glomerular volume, our data showed, for the first time, that the mesangial expansion induced in IgA nephropathy (Fig. 4) stayed unchanged when fish oil alone was used. The significant inhibition of mesangial expansion was clearly seen with α-tocopherol supplementation, in conjunction with either fish oil or corn oil. Indeed, in the latter two groups of IgA nephropathy rats, the glomerular planar areas were no different from those of the control animals. Our data suggest that it is α-tocopherol, rather than fish oil, which modulates the glomerular planar area and glomerular hypertrophy. Our data are consistent with recent findings in streptozotocin diabetic rats(28), in whom the glomerular TGF-β1 and glomerular size were significantly reduced with α-tocopherol dietary supplementation. Severe fibrosis is associated with TGF-β1 overexpression(29), and the data thatα-tocopherol decreases TGF-β1 in diabetes(28) as well as in IgA nephropathy(11) support the potential utility of α-tocopherol in the treatment of these progressive renal diseases.
Oxidative stress has been demonstrated in leukocytes(9), mesangial cells(8) in human IgA nephropathy and in rat macrophages(13) in experimental IgA nephropathy. The severity of proteinuria in patients with IgA nephropathy has been correlated to increased superoxide production(10). Our previous study has shown that the elevated malondialdehyde and TGF-β1 content in IgA nephropathy rat kidneys can be reduced by a specific antioxidant, α-tocopherol(11). In the present study, we have demonstrated thatα-tocopherol, and not fish oil, reduced the expression of the fibrogenic cytokine, TGF-β1, and inhibited glomerular mesangial expansion. We conclude that the beneficial effects of fish oil alone (withoutα-tocopherol supplementation) still remain to be established in this IgA nephropathy model. It is possible that fish oil has a moderating effect on IgA nephropathy and the trend is there but our data on the glomerular planar area or TGF-β1 expression did not achieve statistical difference when fish oil was used alone.
Abbreviations
- BGG:
-
bovine γ-globulin
- TGF-β1:
-
transforming growth factorβ1
- TBHQ:
-
tertiary butylhydroquinone
References
Berger J, Hinglais N 1968 Les depots intracapillaires d'IgA-IgG. J Urol 73: 694–695.
Donadio JV, Grande JP 1989 Immunoglobulin A nephropathy: a clinical perspective. J Am Soc Nephrol 8: 1324–1332.
Galla JH 1995 Perspectives in clinical nephrology. IgA nephropathy. Kidney Int 47: 377–387.
Julian BA, Waldo B, Rifai A, Mestecky J 1988 IgA nephropathy, the most common glomerulonephritis worldwide: a neglected disease in the United States?. Am J Med 84: 129–132.
Cattran DC, Greenwood C, Ritchie S 1994 Long-term benefits of angiotensin-converting enzyme inhibitor therapy in patients with severe immunoglobulin A nephropathy: a comparison to patients receiving treatment with other antihypertensive agents and to patients receiving no therapy. Am J Kidney Dis 23: 247–254.
Scheinman JI, Trachtman H, Lin C-Y, Langman CB, Chan JCM 1997 IgA nephropathy: to treat or not to treat?. Nephron 75: 251–258.
Donadio JV, Bergstralh EJ, Offord KP, Spencer DC, Holley KE 1994 A controlled trial of fish oil in IgA nephropathy. N Engl J Med 331: 1194–1199.
Pei Y, Scholey J, Thai K, Suzuki M, Cattran D 1997 Association of angiotensinogen gene T235 variant with progression of immunoglobulin A nephropathy in Caucasian patients. J Clin Invest 100: 814–820.
Chen A, Chen WP, Sheu LF, Lin CY 1994 Pathogenesis of IgA nephropathy: in vitro activation of human mesangial cells by IgA immune complexes. J Pathol 173: 119–126.
Kashem A, Masayuki E, Nomoto Y, Sakai H, Nakazawa H 1994 FcαR expression on polymorphonuclear leukocyte and superoxide generation in IgA nephropathy. Kidney Int 45: 868–875.
Trachtman H, Chan JCM, Chan W, Valderrama E, Brandt R, Wakely P, Futterweit S, Maesaka J, Ma C 1996 Vitamin E ameliorates renal injury in an experimental model of immunoglobulin A nephropathy. Pediatr Res 40: 620–626.
Emancipator SN, Gallo GR, Lamm ME 1983 Experimental IgA nephropathy induced by oral immunization. J Exp Med 157: 572–582.
Yi ZW, Rodriguez GE, Krieg RJ Jr, Tokieda K, Chan, JCM 1996 Rat macrophages in experimental IgA nephropathy. Biochem Mol Med 57: 52–155.
Chan W, Valerie KC, Chan JCM 1993 Expression of insulin-like growth factor-1 in uremic rats: growth hormone resistance and nutritional intake. Kidney Int 43: 790–795.
Sowell AL, Huff DL, Yeager PR, Caudill SP, Gunter EW 1994 Retinol, α-tocopherol, lutein/zeaxanthin, β-cyptoxanthin, lycopene, α-carotene, trans β-carotene, and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection. Clin Chem 40: 411–416.
Schmitz HH, Poor CL, Wellman RB, Erdman JW 1991 Concentrations of selected carotenoids and vitamins in human liver, kidney and lung tissue. J Nutr 121: 1613–1621.
Bennett WM, Walker RG, Kincaid-Smith P 1989 Treatment of IgA nephropathy with eicosapentaenoic acid: a two-year prospective trial. Clin Nephrol 31: 128–131.
Cheng IKP, Chan PCK, Chan KK 1990 The effect of fish-oil dietary supplement on the progression of mesangial IgA glomerulonephritis. Nephrol Dial Transplant 5: 241–246.
Pettersson EE, Rekola S, Berglund L, Sundqvist KG, Angelin B, Diczfalusky U, Bjorkhem I, Bergstrom J 1994 Treatment of IgA nephropathy with ω-3-polyunsaturated fatty acids: a prospective, double-blind, randomized study. Clin Nephrol 41: 183–190.
Scharschmidt LA, Gibbons NB, McGarry L, Berger P, Axelrod M, Janis R, Ko YH 1987 Effects of dietary fish oil on renal insufficiency in rats with subtotal nephrectomy. Kidney Int 32: 700–709.
Tateno S, Kobayashi Y, Robinson DR 1997 Dietary fish oil supplementation exacerbates serum sickness nephritis in mice. Nephron 77: 86–92.
Clark WF, Parbtani A, Philbrick DJ, Holub BJ, Huff MW 1991 Chronic effects of ω-3 fatty acids (fish oil) in a rat 5:6 renal ablation model. J Am Soc Nephrol 1: 1343–1353.
Bilo HJG, van der Heide JJH, Gans ROB, Donker AJM 1991 Omega-3 polyunsaturated fatty acids in chronic renal insufficiency. Nephron 57: 385–393.
Purkerson ML, Joist JH, Valdes A, Yates J, Morrison A, Klahr S 1985 Inhibition of thromboxane synthesis ameliorates the progressive kidney disease of rats with subtotal ablation. Proc Natl Acad Sci USA 82: 193–197.
Diaz MN, Frei B, Vita JA, Keaney JF 1997 Antioxidants and atherosclerotic heart disease. N Engl J Med 337: 408–416.
McGrath LT, Brennan GM, Donnelly JP, Johnston GD, Hayes JR, McVeigh GE 1996 Effect of dietary fish oil supplementation on peroxidation of serum lipids in patients with non-insulin dependent diabetes mellitis. Atherosclerosis 121: 275–283.
Cho SH, Im JG, Choi YS, Son YS, Chung MH 1995 Lipid peroxidation and 8-hydroxydeoxyguanosine formation in rats fed fish oil with different levels of vitamin E. J Nutr Sci Vitaminol 41: 61–72.
Craven PA, DeRubertis FR, Kagan VE, Melhem M, Studer RK 1997 Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-β, and glomerular size in diabetes. J Am Soc Nephrol 8: 1405–1414.
Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J 1997 Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776.
Acknowledgements
The authors thank Betty Timozek for secretarial assistance, Rosalind Bradley, M.Div., for editorial assistance and Linda Benson, M.P.H., and Kenley Ward, B.A., for laboratory assistance.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health T32 DK07526 and R01 DK50419. Hoffmann-LaRoche Inc., Nutley, NJ, funded the α-tocopherol measurements.
James C. M. Chan, M.D., Nephrology Division, Department of Pediatrics, Virginia Commonwealth University's Medical College of Virginia, P.O. Box 980498, Richmond, VA 23298–0498.
Rights and permissions
About this article
Cite this article
Kuemmerle, N., Chan, W., Krieg, R. et al. Effects of Fish Oil and α-Tocopherol in Immunoglobulin A Nephropathy in the Rat. Pediatr Res 43, 791–797 (1998). https://doi.org/10.1203/00006450-199806000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199806000-00012