Abstract
The incidence of venous thromboembolic disease is reduced in children compared with adults. Thromboprotective mechanisms, some of which have already been identified in plasma, must be present in children. Blood vessel walls have important antithrombotic properties that maintain blood fluidity. This is in part due to proteoglycan (PG)-related glycosaminoglycan (GAG) molecules within vessel walls. PGs are macromolecules with covalently attached GAG chains, either chondroitin, dermatan, heparan, or keratan sulfate. The influence of age on the concentration and anticoagulant activities of PGs and GAGs, within vein walls before puberty has not been previously investigated. We hypothesized that developmental differences in vein walls may contribute to the reduced risk of thrombosis in children. We used a rabbit model to examine morphologic and biochemical features of inferior venae cavae (IVCs). We assessed IVC wall morphology, PG distribution, GAG mass, and GAG antithrombin activity. Morphologically, there were only minor differences between pups and adult rabbits' IVCs. However, there was a significant increase in GAGs by mass in IVCs from pups compared with adult rabbits (p = 0.012). In addition the total antithrombin activity (p = 0.04), and especially that of heparan sulfate (p = 0.01) was significantly increased in pups compared with adult rabbits. These results demonstrate important differences in the antithrombotic properties of IVC walls in pups and adult rabbits. In summary, developmental differences in vein wall PG content and activity exist which may contribute to the reduced risk of venous thromboembolism in children. Further characterization of these differences is required.
Similar content being viewed by others
Main
Clinically, the incidence of venous thromboembolic disease is significantly decreased during infancy(1) and childhood(2) compared with adults, suggesting that protective mechanisms are in place. Virchow's triad states that the risk of developing a thrombosis is related to the hemostatic system, vessel wall, and blood flow(3). Selected aspects of the hemostatic system have been investigated in the young, and some potentially important age-dependent protective mechanisms have been delineated(4–7). However, there is virtually no information on the influence of age (prepubescence) on the antithrombotic properties of vein walls. PGs are macromolecules with covalently attached GAG chains that have antithrombotic properties. PGs on endothelial cell surfaces(8) and in basement membranes of the intimal layer of veins have a profound influence on the pathogenesis of venous thromboembolic events in adults. Several studies have shown an age dependence of PG anticoagulant activities in a variety of different locations and diseases(9). Therefore we designed studies to determine whether prepubertal age influenced PG content and anticoagulant activities in the vein wall.
Studies of vessels from autopsy samples are not ideal because of postmortem changes, and/or adverse influences of techniques used to preserve tissues. Adult rabbits have been studied previously for aortic GAG content(10) and also PG distribution after de-endothelialization(11–13). Therefore we chose the rabbit model to determine whether there were age-dependent morphologic differences between the intima and media, and the amount, type and anticoagulant activities of GAGs present in the IVC. Our studies showed that there were increased concentrations and anticoagulant activities of GAGs in IVCs of pups compared with adult rabbits.
METHODS
Animal Model
The effect of age on components of the IVC wall was investigated in New Zealand White prepubertal pups (0.38 ± 0.05 kg) and adult rabbits (mean weights of 2.79 ± 0.72 kg)(14). All experiments were carried out with prior approval from the Animal Ethics Board of McMaster University, and performed on the same day that the animals arrived at the Central Animal Facility at McMaster University.
Vessel Wall Morphology
Animal and tissue preparation. An established method for perfusion-fixation of the entire vascular system, including the IVC, was used(15). In brief, after induction with isoflurane, rabbits were anesthetized, and the abdominal aorta was isolated. Appropriate sized tubing was inserted up to the aortic arch, and after perfusing the vascular system with Krebs-Henseleit solution the tissues were fixed by switching the perfusate to glutaraldehyde solution. Upon completion of perfusion-fixation, the IVCs were removed and placed in a glutaraldehyde solution at 4°C for up to 4 h before processing. IVCs were placed on a wax board, and the external mesentery was removed. Full circumference 1-mm sections were taken for light microscopy, TEM, and SEM.
SEM. Segments for SEM were opened longitudinally along the IVCs and the adventitial surfaces glued to coverslips with Permabond 910 (Permabond Industrial, Englewood, NJ). After immersion in 1% osmium tetroxide:0.2 M sodium cacodylate buffer (vol/vol, 1:1) for 2 h, ethanol dehydration and drying to the critical point (Ladd critical-point drier, Ladd Research Industries, model 2800, Burlington, VT); segments were gold sputter-coated(Polaron Instruments Inc., Hatfield, PA) before SEM (Phillips 501B instrument, Phillips, Eindhoven, Holland).
Light Microscopy and TEM. All electron-dense stains used were from Marivac Ltd., Halifax, Nova Scotia, Canada. Full circumferential rings were stained for GAGs with a ruthenium red solution (1.5 mg/mL in 0.2 M sodium cacodylate buffer) followed by staining with a 1:1 mixture of ruthenium red:OsO4 (0.75:10 mg/mL) at 4°C for 2 h. After rinsing with distilled water and staining en bloc with 2% aqueous uranyl acetate, the samples were dehydrated with serial ethanol solutions. After dehydration, the samples were treated with a propylene oxide/Spurr resin (Marivac Ltd.) mixture and finally placed in 100% Spurr resin for 8 h before embedding in Beem capsules (Marivac Ltd.). The resin was polymerized (65°C for 6-8 h), and 1-μm thick, full circumference sections were cut. After staining with a 1% toluidine blue solution (Marivac Ltd.) and mounting on glass slides using Permount (Fischer Scientific, Toronto, Ont.); areas of the IVCs for TEM were selected by LM and ultrathin sections (30 nm) cut by microtome for mounting on copper grids (200 mesh) for TEM (JEM-1200EX, JEOL, Peabody, MA).
Morphologic Evaluation
SEM morphology. SEM micrographs (640-fold magnification) were examined for morphologic evidence of endothelial cell stimulation, which includes the presence of blood elements, evidence of mitotic events, and alterations in the surface morphology of the endothelium.
SMC cell ultrastructure by TEM. Samples were analyzed for morphologic differences in the intimal layers of IVCs using TEM. Two micrographs were used for each sample (2500 × magnification). Micrographs were examined to see if there were any phenotypic differences in SMCs from the internal elastic lamina to the third SMC layer. SMCs in vessel walls are described as having secretory or contractile phenotype(16). Two sections for each pup (n = 5) and adult rabbit (n = 5) were analyzed for a total of 39 pup and 53 adult rabbit SMC samples(16).
Distribution of SMCs and connective tissues. Ten micrographs(25 000 magnification) were taken from each of two sections from five pups and five adult rabbits. In each case, the content of SMCs, elastin, collagen, and ECM containing PG were determined using a digitizing board (Summasketch II, model MM II 1201, Summagraphics, Seymour, CT) and image analysis software(version 1.20.09, Jandel Scientific, Corte Madera, CA). Areas of the tissue components were expressed as a percent of intimal or medial space in the micrograph, and data from each animal were averaged.
PG Evaluation
Enzyme degradation. Verification of PG identity based on location was evaluated by determining their susceptibility to degradation by GAG-specific enzymes from three pups and one adult rabbit. Animals were euthanized with sodium pentobarbital (65 mg/kg), and the IVCs were removed and cut into 1-mm rings, which were then cut in half. The segments were exposed to one of the following enzyme treatments for 20 min at 37°C: 1) 0.25 M Tris-HCl buffer pH 7.6; 2) 2.5 U/mL chondroitinase ABC (ICN Biochemicals, Costa Mesa, CA), which was specific for CS and DS; 3) 2.5 U/mL chondroitinase AC (ICN Biochemicals), specific for CS; 4) 0.05 U/mL chondroitinase B (ICN Biochemicals), specific for DS; and 5) 0.1 M nitrous acid, specific for degradation of HS. After exposure to the GAG degrading enzymes, the tissues were fixed (2% glutaraldehyde or 4% paraformaldehyde).
PG distribution and diameter. PG distribution was measured in the intima from pups (n = 5) and adult rabbits (n = 5). PGs from 10 micrographs (× 25 000) from two sections were counted as either CSPGs, DSPGs, or HSPGs, depending on their ECM location. PGs were expressed as the number of PGs/μm(2) of ECM containing PG.
Extraction and mass analysis of GAGs. The procedure for extraction of GAGs has been reported previously(10). Briefly, isolated venae cavae samples were minced and delipidated with methanol-chloroform. GAGs were removed from the remaining tissue by base elimination (NaOH/NaBH4). The mass of extracted GAGs was determined using 7.5% SDS-PAGE(17). DS (Sigma Chemical Co.) was used as a standard. Each GAG extraction sample was reconstituted in 1 mL of distilled water, and 5-μL aliquots were treated with either 5 μL of saline, 1 μL of chondroitinase B (0.0001 U), 1 μL of chondroitinase ABC(0.005 U), or 1 μL of chondroitinase ABC (0.005 U) + 1 μL of heparitinase (0.0001 U); each made up to a total volume of 10 μL with saline. After heating for 2 h at 37°C, SDS-PAGE of each sample was carried out under reducing conditions. Gels were stained for GAGs with alcian blue and silver stain(18), dried in Gel wrap (Biodesign, New York, NY), and analyzed by laser densitometry (LKB Ultroscan XL, model LKB-2222, LKB Ltd., Bromma, Sweden). The area under the densitometry curves was calculated in the same way as areas on the micrographs.
Analysis of antithrombin activity. The lyophilized GAGs were analyzed for antithrombin activity by measuring inhibition of thrombin using a method described previously, with slight modifications(19). Briefly, 40 μL of reconstituted samples were heated at 37°C for 30 min with 30 μL of saline + 10 μL of either 0.01 M sodium acetate or a solution containing a GAG degrading enzyme. After heating with 10 μL of defibrinated plasma (obtained by winding out the clot formed in plasma treated with 0.17 U of Arvin/mL) for 1 min at 37°C, 10μL of 15 U of purified human thrombin (Enzyme Research Labs, South Bend, IN)/mL saline were added followed by a further 1-min incubation. A clock was started as 100 μL of adult pooled plasma, preheated to 37°C, was added, and the time of the first appearance of a clot at the end of a wire loop was recorded. The prolongation of clot times was compared with clot times observed with similar reaction mixtures containing known amounts of DS (Sigma Chemical Co.) used to produce a standard. Results were calculated to reflect the activities of GAGs/mg of tissue.
Statistical analysis. All data are expressed as mean ± 1 SEM. The data from the TEM morphologic examinations were compared with a one-way ANOVA. The data from the GAG extraction analysis and antithrombin assays were compared using a general linear model ANOVA, after normalization by a rank transformation.
RESULTS
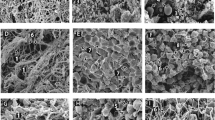
Endothelial cells. SEM showed that endothelium from IVCs was similar within each of the two experimental groups (Fig. 1). White and red blood cells were observed on the endothelium of both pups and adult rabbits. Endothelial cells from pups appeared less spread out than endothelial cells from adult rabbits, and cells from pups showed a more prominent nuclear bulge. One notable difference in the endothelium from pups was the presence of raised cells.
Subendothelial layer morphology and composition. The composition of IVCs from pups and adult rabbits is illustrated in Figure 2a. Components of IVC walls were not significantly different between pups and adult rabbits. Elastin was consistently present in the subendothelial ECM, whereas collagen was always observed below the thin layer of SMCs. SMCs in IVCs from pups and adult rabbits were consistently observed to have contractile phenotypes.
IVC intimal SMC and connective tissue content(a) and PG distribution (b). The average values of data from micrographs from pups (n = 34) and adult rabbits (n = 39) are shown. Error is 1 SE of the mean. Significant differences (*) between pup and adult samples were found for total PG and CSPG (p < 0.05).
The concentrations of ECM PGs in IVCs from pups and adult rabbits are illustrated in Figure 2b. The concentrations of total PGs and CSPGs were significantly increased in IVCs from pups (p < 0.05). There were no significant differences in the concentrations of HSPGs between pups and adult rabbits. DSPGs were not identified in the subendothelial matrix of pups or adult rabbits.
GAG content of the IVC. Enzyme degradation verified the presence of GAGs in the rabbit IVC. In fact treatment with GAG-specific enzymes led to loss of structural integrity of the IVC sections.
The concentrations of GAGs in IVCs from pups and adult rabbits are shown in Table 1. The mass of GAGs (consisting entirely of DS) was significantly increased in pups compared with adult rabbits (p = 0.012). All of the enzyme treatments completely degraded GAGs in IVC samples for both experimental groups.
GAG antithrombin activity in the IVC. The antithrombin activities of GAGs from IVCs from pups and adult rabbits are shown in Table 2. The antithrombin activities of GAGs were increased in pups compared with adult rabbits (p = 0.040). The increased antithrombin activity in pups was primarily due to HS (p = 0.01).
DISCUSSION
The observation that infants and children have a significantly decreased incidence of venous thromboembolic complications, compared with adults, suggests that there are age-dependent protective mechanisms present. Some potentially important age-dependent protective mechanisms in plasma components of hemostasis have been delineated(7, 20, 21). However, the influence of age on the concentration and anticoagulant activities of PGs and GAGs in vein walls has not been studied previously in the prepubertal state. The objective of our study was to compare the morphology of IVC walls, and the presence, distribution, and antithrombin activities of PGs and GAGs in IVCs from prepubertal pups and adult rabbits. The results showed that although minimal structural differences exist between pups and adult rabbits, the concentration and antithrombin activities of GAGs in the IVCs are significantly increased in rabbit pups. These physiologic differences may provide another important protective mechanism from thromboembolic events in the young compared with adults.
Population-based studies in Canada report that the incidence of venous thromboembolic disease in children is 0.0007% per year(2). In newborns the incidence is 0.24% per year among all admissions to neonatal intensive care units(1). In contrast, between 3 and 5% of adults will suffer from at least one venous thrombotic event during their lifetime(22). The reduced incidence of venous thromboembolic disease during early childhood applies even in the presence of congenital prethrombotic disorders(2), or acquired risk factors such as orthopedic surgery(2). These age-dependent differences strongly suggest that there are mechanisms in place that protect children from venous thromboembolic disease.
Selected aspects of the hemostatic system have been investigated to assess the influence of age on the formation and regulation of fibrin clots during early life(23). The generation of thrombin, a key enzyme in blood coagulation because it cleaves fibrinogen with subsequent fibrin formation, is reduced by 50% at birth(20) and by 20% throughout childhood(7). Decreased plasma concentrations of the vitamin K-dependent proteins, particularly prothrombin, are responsible for the decreased capacity to generate thrombin during childhood(20). The generation of thrombin and thrombin's activities are regulated by several inhibitory systems. Antithrombin, heparin cofactor II, and α2M are plasma inhibitors that complex with thrombin, neutralizing its coagulant activities(21, 24, 25). Although antithrombin is the major inhibitor of thrombin in plasma from adults, α2M has an increased capacity to inhibit thrombin throughout childhood, reflectingα2M's increased plasma concentration(21). Thrombin's coagulant activities are also regulated by the protein C/protein S/TM pathway(26, 27). In vivo, thrombin binds to TM, an endothelial cell surface receptor. Thrombin complexed to TM cleaves protein C, converting it to its active form, APC, which in the presence of protein S, functions as an inhibitor of coagulation by proteolysis of FVa and FVIIIa(28). Plasma concentrations of TM are increased at birth and throughout childhood, which may reflect increased endothelial cell expression of TM(29, 30). In summary, decreased thrombin generation, and enhanced inhibition of thrombin byα2M provide at least two mechanisms that protect children from thromboembolic events(31).
The influence of age on antithrombotic properties of veins in the very young has not been assessed previously. Morphologically, veins are composed of intimal, medial, and adventitial layers(32). The intimal layer lines the lumen and consists of a layer of endothelial cells. These cells are supported by a basement membrane consisting of a continuous sheet of ECM components, including fibronectin, laminin, collagen, and certain PGs. The intimal layer extends to the luminal surface of the internal elastic lamina, a dense sheet of elastic fibers. The internal elastic lamina is not well defined in vein walls, and therefore the intimal layer is demarcated by the first layer of SMC. The medial layer of IVCs contains three or four layers of SMCs, which are not separated by elastic tissue and an extensive layer of collagen. The adventitial layer is mainly composed of collagen and fibroblasts.
In this study, SEM revealed minor differences between the endothelium from pups and adult rabbits. Raised cells were seen only in pups. Neither the intimal SMC content nor phenotype of IVCs were significantly different between pups and adult rabbits. Collagen is a major component of the ECM, and a potent activator of both platelets and the coagulation system. The concentration and distribution of collagen in arterial walls changes with increasing age(33). However, no previous studies have examined vein walls. In our study, collagen was seen in similar ratios in the intimal ECM in pups and adult rabbits. Elastin was also seen in similar ratios in pups and adult rabbits. The similarity in structure of IVCs from pups and adult rabbits in our study suggests that the veins of pups are structurally mature.
The presence of PGs was verified using GAG-specific degrading enzymes. Treatment with these enzymes led to loss of structural integrity of the IVC sections, highlighting the importance of PGs in the ECM. PGs were identified based on their ECM location(34, 35). CSPGs in the subendothelial ECM are dispersed among the interstitial elastin, HSPGs are located in the endothelial basement membranes and DSPGs are contained strictly in the medial layer. There were significant differences in the total concentrations of PGs and CSPGs between pups and adult rabbits. HSPG concentrations were not significantly different in the two groups and DSPGs were absent from the subendothelial matrix.
The only GAG detected by mass analysis was DS. Degradation with chondroitinase B (specific for DS) completely removed all detectable GAG. Therefore, the proportion of total GAG by a mass that was present as CS and HS chains was less than 5%, which is below the sensitivity of the assay. The measurable mass of GAGs was only 0.1 times the concentration found in aortas(E. Nitschmann, unpublished observations). Overall GAG antithrombin activity was increased in pups compared with adult rabbits, reflecting primarily significant increases in HS. CS showed a trend toward increased activity in the pups; however, it did not reach statistical significance. The lack of correlation between GAG concentration by mass analysis and GAG activity was not surprising and has been shown previously in studies using fetal distal lung epithelium(19). The antithrombin activity of GAGs reflects the number of antithrombin and heparin cofactor II binding sites per GAG molecule, which is not directly proportional to GAG mass(19).
The evidence that HS had increased antithrombin activity in pups may indicate that HS from young animals contains more pentasaccharide sequences necessary for binding antithrombin. The HS was extracted from the whole IVC and, therefore, represents HS on both the luminal and abluminal surfaces of the endothelium as well as that present in SMC basement membranes. Extrapolating from the in vitro antithrombin data to inhibition of thrombin on the endothelial surface may not be possible. However, these data suggest that pups may have an increased capacity to inhibit plasma thrombin if HSPGs are located on the luminal surface of the endothelium or if the ECM is exposed to thrombin.
The antithrombin activity of GAGs from IVCs was increased by 5-fold compared with the activity of GAGs present in rabbit thoracic aorta (E. Nitschmann, unpublished observations). Veins have lower vascular pressure, potentiating stasis of blood, which promotes the formation of thrombin at the vessel wall. Therefore, increased antithrombin activity in vein walls compared with arterial walls may be a necessary protective mechanism.
In summary, this is the first study to examine the developmental differences in the structure and antithrombotic properties of veins, comparing prepubertal pups with adult rabbits. Morphologically, only minor differences existed between pups and adult rabbits, which suggested that veins achieve structural maturity at an early age. Mass analysis showed increased amounts of DS in pups compared with adult rabbits. However, functional analysis showed increased antithrombin activity primarily due to HS. This study demonstrated the presence of important age related thromboprotective mechanisms in vein walls of prepubertal pups compared with adult rabbits. Further characterization of these differences is necessary to explore their full implications.
Abbreviations
- PG:
-
proteoglycan
- GAG:
-
glycosaminoglycan
- IVC:
-
inferior vena cava
- TEM:
-
transmission electron microscopy
- SEM:
-
scanning electron microscopy
- SMC:
-
smooth muscle cell
- ECM:
-
extracellular matrix
- CS:
-
chondroitin sulfate
- DS:
-
dermatan sulfate
- HS:
-
heparan sulfate
- α2M:
-
α2-macroglobulin
- TM:
-
thrombomodulin
References
Schmidt B, Andrew M 1995 Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics 96: 939–943
Andrew M, David M, Adams M, Ali K, Anderson R, Barnard D, Berstein M, Brisson L, Cairney B, DeSai D 1994 Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood 83: 1251–1257
Virchow R, ed 1856 Gesammelte Abhandlungen zur wissenschaftlchen Medicin. Von Meidinger, Phlogose und Thrombose in Gefaesystem, Frankfurt, pp 458–636
Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen D, Powers P 1987 Development of the human coagulation system in the full-term infant. Blood 70: 165–172
Andrew M, Paes B, Johnston M 1990 Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol 12: 95–104
Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen D, Castle V, Powers P 1988 Development of the human coagulation system in the healthy premature infant. Blood 72: 1651–1657
Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L 1992 Maturation of the hemostatic system during childhood. Blood 80: 1998–2005
Hatton M, Moar S, Richardson M 1988 Evidence that rabbit 125-I-antithrombin III binds to proteoheparan sulphate at the subendothelium of the rabbit aorta in vitro. Blood Vessels 25: 12–27
Watanabe K, Oohira A, Uramotot I, Totsuka T 1986 Age-related changes in the content and composition of glycosaminoglycans isolated from the mouse skeletal muscle: Normal and dystrophic conditions. J Biochem 100: 167–173
van der Heiden R, Hatton MWC, Moore WJ 1988 Extraction and analysis of glycosaminoglycans from intima-media of single aortae: effect of balloon catheter de-endothelialization on the content and profile of glycosaminoglycans. Atherosclerosis 73: 203–213
Richardson M, Kinlough-Rathbone R, Groves H, Jorgensen L, Mustard J, Moore S 1984 Ultrastructural changes in re-endothelialized and non-endothelialized rabbit aortic neo-intima following re-injury with a balloon catheter. Br J Exp Pathol 65: 597–611
Moore S, Richardson M 1985 Proteoglycan distribution in catheter-induced aortic lesions in normolipaemic rabbits. Atherosclerosis 55: 313–330
Richardson M, Hatton M 1993 Transient morphological and biochemical alterations of arterial proteoglycan during early wound healing. Exp Mol Pathol 58: 77–95
Weisbroth SH, Flatt RE, Kraus AL, eds 1974 Biology of the Laboratory Rabbit. Academic Press, New York, pp 50–69
Richardson M, Hadcock SJ, Hatton BD, Winocour PD, Hatton MWC 1993 Proteoglycan alterations in the aortic intima-media of alloxan-diabetic rabbits: an ultrastructural and biochemical study. Exp Mol Pathol 58: 145–159
Campbell GR, Campbell JH 1985 Smooth muscle phenotypic changes in arterial wall homeostasis: Implications for the pathogenesis of atherosclerosis. Exp Mol Pathol 42: 139–162
Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Moller HJ, Heinegard D, Poulsen JH 1993 Combined alcian blue and silver staining of sub-nanogram quantities of proteoglycans and glycosaminoglycans in sodium dodecylsulfate-polyacrylamide gels. Anal Biochem 209: 169–175
O'Brodovich H, Berry L, D'Costa M, Burrows R, Andrew M 1991 The influence of fetal pulmonary epithelium on thrombin activity. Am J Physiol 5:L262–L270
Andrew M, Schmidt B, Mitchell L, Paes B, Ofosu F 1990 Thrombin generation in newborn plasma is critically dependent on the concentration of prothrombin. Thromb Haemostasis 63: 27–30
Schmidt B, Mitchell L, Ofosu F, Andrew M 1989 α2-Macroglobulin is an important progressive inhibitor of thrombin in neonatal and infant plasma. Thromb Haemostasis 62: 1074–1077
Salzman EW, Hirsh J, Coleman RW, Hirsh J, Marder VJ, Salzman EW, eds 1994 The epidemiology, pathogenesis, and natural history of venous thrombosis. In: Hemostasis and Thrombosis: Basic Principles, 3rd Ed. JB Lippincott, Philadelphia, pp 1275–1296
Andrew M, Brooker L, Paes B, Weitz J 1992 Fibrin clot lysis by thrombolytic agents is impaired in newborns due to a low plasminogen concentration. Thromb Haemostasis 68: 325–330
Andrew M, Mitchell L, Ofosu F α2-Macroglobulin and antithrombin III are equally important inhibitors of thrombin during childhood. Blood 62: 380( abstr)
Mitchell L, Hoogendoorn H, Paes B, Giles A, Andrew M 1993 Hereditary type II α2-macroglobulin (α2M) deficiency. Thromb Haemostasis 69: 113
Dahlback B 1991 Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemostasis 66: 49–61
Dahlback B 1995 The protein C anticoagulant system: inherited defects as basis for venous thrombosis. Thromb Res 77: 1–43
Monnens LAH, Retera RJM 1967 Thrombotic thrombocytopenic purpura in a neonatal infant. J Pediatr 71: 118–123
Orbe I, Paramo JA, Pinacho A, Hermide J, Rocha E 1995 Plasma thrombomodulin is increased in cord blood of healthy newborns. [letter] Thromb Haemostasis 73: 326
Aurousseau M, Amiral J, Boffa M 1991 Level of plasma thrombomodulin in neonates and children. Thromb Haemostasis 65: 1232
Mitchell L, Piovella F, Ofosu F, Andrew M α2-macroglobulin may provide protection from thromboembolic events in antithrombin III deficient children. Blood 78: 2299–2304
Rhodin J 1974 Histology. In: The Cardiovascular System. Oxford University Press, New York, pp 332–370
Bendeck MP, Keeley FW, Langille BL 1994 Perinatal accumulation of arterial wall constituents: relation to hemodynamic changes at birth. Am J Physiol 267:H2268–H2279
Iozzo RV, Cohen IR, Grassel S, Murdoch AD 1994 The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J 302: 625–639
Scott JE 1990 Proteoglycan: collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J Anat 169: 23–35
Author information
Authors and Affiliations
Additional information
Supported by Project 7 of the Medical Research Council of Canada Groups in Developmental Lung Biology. M.A. holds a Career Investigator Award from the Heart and Stroke Foundation of Canada.
Rights and permissions
About this article
Cite this article
Nitschmann, E., Berry, L., Bridge, S. et al. Morphologic and Biochemical Features Affecting the Antithrombotic Properties of the Inferior Vena Cava of Rabbit Pups and Adult Rabbits. Pediatr Res 43, 62–67 (1998). https://doi.org/10.1203/00006450-199801000-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199801000-00010