Abstract
N-Methyl-D-aspartate (NMDA) receptors are a calcium-conducting class of excitatory amino acid receptors that are involved in neuronal development and migration. Certain well known teratogens (e.g. homocysteine, ethanol, and chloroform) that induce congenital neural tube and neural crest defects also have the capacity to act as NMDA receptor antagonists. We hypothesized that teratogenicity was a general property of NMDA receptor antagonists, and that high affinity NMDA receptor antagonists would induce neural tube and neural crest defects. Chicken embryos were given 5, 50, or 500 nmol/d of selected NMDA receptor antagonists for 3 consecutive days during the process of neural tube closure, beginning 4 h after the beginning of incubation. Selected NMDA receptor antagonists represented three classes of antagonists: ion channel blockers, glycine site antagonists, and glutamate site agonists and antagonists. All classes of NMDA receptor antagonists induced embryonic death and congenital defects of the neural crest and neural tube; however, the channel blockers were the most potent teratogens. Dextromethorphan at 500 nmol/embryo/d killed more than half the embryos and induced congenital defects in about one-eighth of the survivors; dextromethorphan was also highly lethal at 50 nmol/embryo/d. Glutamate site NMDA receptor agonists (NMDA and homoquinolinic acid) displayed weak toxicity relative to their known NMDA receptor potency. Taken together, these data indicate that NMDA receptor antagonists, particularly channel blockers, are potent teratogens in the chicken embryo model. Because dextromethorphan is a widely used nonprescription antitussive, its strong teratogeneticity using this model is particularly noteworthy.
Similar content being viewed by others
Main
Congenital defects typically occur within sets or associations that have been described by statistical evaluation of epidemiologic data. One common association that has been validated by numerous epidemiologic and experimental studies is the relationship among craniofacial, CNS, and heart defects. The biologic basis of this relationship is straightforward: the mesenchyme of the face and the conotruncal septum of the heart are derived from cranial neural crest cells, the CNS is derived from the neural tube, and the neural crest is both geographically contiguous and functionally interchangeable with the neural tube in the early embryo(1). Thus a teratogenic agent that inhibits neural crest migration to produce facial or septal defects also would be expected to inhibit neural tube closure, and conversely.
Among the numerous factors that have been shown to be associated with congenital neural crest and neural tube defects are three that occur most frequently in the literature: folic acid deficiency, ingestion of ethanol, and exposure to environmental toxins. These three factors in aggregate may account for the majority of neural crest and neural tube defects that cannot be accounted for by the 22q11 deletion, Down's syndrome, or other known genetic factors(2). No common biologic basis for their common dysmorphogenetic effect has been reported for these three seemingly disparate teratologic agents. However, the unifying mechanism may be their shared ability to perturb the function of the NMDA family of glutamate receptors.
One of the most obvious and fully described effects of folic acid deficiency is a rise in the concentration of serum homocysteine, an amino acid that is produced in the metabolism of methionine(3, 4). Recently, we showed that homocysteine induces neural crest and tube defects in a time- and concentration-dependent fashion(5). At concentrations that correlate with the induction of neural crest and tube defects, homocysteine also acts as an NMDA receptor antagonist at the glycine site(6, 7). This ability to act as an NMDA receptor antagonist is shared by ethanol(8–11), and recent studies report that the teratogenic action of ethanol on neural tube derivatives is best understood when it is viewed as a consequence of this effect(12). Furthermore, aliphatic hydrocarbons that are teratogenic contaminants of water also induce craniofacial and neural tube closure defects(13–15). Chloroform and trichloroethylene are the most common of the halogenated hydrocarbons that occur as water pollutants and also are used as anesthetics. Recent evidence has shown that the anesthetic activity of these compounds is related to their ability to act as NMDA receptor antagonists(16–20).
From these data, it may be hypothesized that the ability to induce neural crest and neural tube defects is a general property of NMDA receptor antagonists. In this study we have examined the ability of different classes of NMDA receptor antagonists to cause teratogenic effects in chick embryos. NMDA receptors are dual gated ion channel receptors in that both glutamate and glycine binding are required for receptor activation, which opens a calcium/sodium/potassium-permeable channel. For this study we chose antagonists that act at the glycine or glutamate binding sites or antagonists that bind within the open ion channel (channel blockers). A teratogenic effect would have increasingly serious implications as the clinical use of NMDA agonists and antagonists increases. Of special concern is dextromethorphan, an orally active NMDA antagonist(21, 22), that is widely used as an over-the-counter antitussive medication. The results presented here show that, in avian embryos, NMDA receptor antagonists are potent inducers of neural crest and tube defects, acting in a dose-dependent fashion.
METHODS
Materials. Fertilized chicken eggs were purchased in bulk from HyLine International, Inc. (Dallas Center, IA) and kept refrigerated(14°C) until use. NMDA receptor antagonists were purchased from Tocris Cookson, Inc. (St. Louis, MO), Alexis Biochemicals (San Diego, CA), or else from Sigma Chemical Co. (St. Louis, MO). All stock solutions were made by dissolving drugs in 0.9% saline, and working dilutions for all drugs were prepared for these experiments using 0.9% saline in Falcon 15-mL tissue culture tubes and kept frozen (-20°C) until use.
Negative controls. The following groups were used as negative controls: untreated embryos; sham-treated embryos that were given only the 0.9% saline vehicle, and embryos treated with (-)MK-801.
Positive controls. Our experience has shown that avian embryos may demonstrate seasonal, genetic, or other variations in susceptibility to the experimental induction of developmental defects. To ensure that the susceptibility of these embryos to NMDA antagonists was comparable to that of earlier studies(5), and to provide a comparison defect rate, a set of embryos was treated with 5 μmol/d homocysteine given at the same times as the other NMDA receptor antagonists.
Experimental drugs. Antagonists. NMDA receptor antagonists selected for this study were: channel blockers,(+)MK-801, dextromethorphan, and memantine; glycine site antagonists, kynurenic acid, 7-chloro-kynurenic acid, and 7-chlorothiokynurenic acid; glutamate site antagonists, CPP, D-AP5, and the L-isomer of D-AP5 (L-AP5, an NMDA receptor antagonist known to have a much lower potency than D-AP5 in adult brain NMDA receptors).
Agonists. Selected NMDA receptor agonists included NMDA itself and HQ.
Treatment of embryos. Embryos in ovo were divided into groups of 18 and preincubated in a rocking humidified incubator set at 39°C for 4 h. Embryos in ovo then were removed from the incubator, and the first dose (50 μL in 0.9% saline) was administered to each treatment group, which is a typical dose size for experiments wherein drugs or micronutrients are delivered to avian embryos(5). Immediately after treatment of embryos, the eggs were resealed using a small amount of melted paraffin wax and returned to the incubator. Similar treatments were given at 24 and 48 h after this time, or after 28 and 52 h of total incubation time, respectively. At 24 h after the last treatment (76 h total incubation time), the embryos were were stained in ovo with 0.02% neutral red stain in 0.9% saline. Five minutes later eggs were visualized under a dissection microscope and examined for developmental abnormalities. Some embryos showing exemplary defects were prepared for observation with scanning electron microscopy.
Statistical analysis. At least three separate experiments were performed with each concentration of a drug, with 18 embryos in each treatment group. Developmental defects were assigned to the following categories:“spinal” including spinal dysraphism and caudal dysgenesis;“craniofacial” including dysraphism, partial or complete absence of facial development, small or missing eyes, or bilateral facial asymmetry;“multiple” including any combination of craniofacial and spinal defects; and “other” or any obvious defect not included in the neural crest or neural tube categories (e.g. very small or very large tubular heart). The total number of defects in each category was normalized to the total number of surviving embryos. Similarly, dead embryos were counted from each data group. All data were then entered into an electronic database using Excel 6.0 (Microsoft, Inc.) and Prism 2.0 (GraphPad, Inc.) and normalized to numbers of defects or deaths per 100 embryos. Data shown are the average ± SEM of values determined for each drug and dosage level among all experiments. Where comparisons of means were performed, determinations of significance were performed using a single-factor ANOVA followed by Fisher post hoc analysis, using Statview 2.0 (Abacus Concepts, Inc.).
RESULTS
Spontaneous developmental defects occurred at a low rate in the untreated embryos, with an average of 0.97 ± 0.41 total observable defects per 100 sham-treated embryos. The number of spontaneous deaths in vehicle-treated groups was also low, 1.46% ± 0.52%. Differences in defect and mortality rates between sham-treated and untreated control embryos were negligible (data not shown). Treatment of embryos with 5 μmol/d DL-homocysteine thiolactone resulted in a total neural tube defect rate of 27.7 ± 3.4 neural tube defects/100 embryos, and a mortality of 46.75 ± 2.7%, confirming a previous study(5).
Channel blockers. Exposure to NMDA receptor channel blockers(+)MK-801, dextromethorphan, or memantine all induced a significant increase in the incidence of craniofacial and spinal developmental lesions(Fig. 1, top). The (-)-stereoisomer of MK-801, a drug known to posses at least an order of magnitude lower potency at blockade of adult brain NMDA receptors, was inactive up to 500 nmol/embryo/d, consistent with the hypothesis that these drugs act to induce neural tube defects by inhibition of NMDA receptor function.
Effect of channel blocker NMDA receptor antagonists on incidence of developmental defects and mortality in developing chicken embryos. During the first 2 d of development chicken embryos (groups of 18) were exposed to 0.5-500 nmol/embryo/d of various NMDA receptor channel blockers as described in “Methods.” After 76-h development, embryos were examined for gross developmental abnormalities (top) or death(bottom). Data represent means ± SEM for defect and mortality rates in at least three separate experiments. Asterisks denote significance(*p < 0.05, **p < 0.01, *** p < 0.001vs vehicle control group). DXM, dextromethorphan.
Only the channel blockers increased the mortality rate to a significant degree (Fig. 1, bottom). At 500 nmol/embryo/d, dextromethorphan and memantine treatments each resulted in a significant increase in mortality. In particular, dextromethorphan treatment resulted in 56.7 ± 10.6 deaths/100 embryos (p < 0.001 versus control embryos). Dextromethorphan was unique among all the drugs examined in this study in that it caused a significant increase in mortality when it was given in doses as low as 50 nmol/embryo/d (14.1% ± 10.7% embryos dead,p < 0.05 versus control).
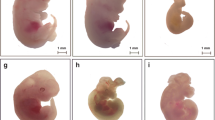
The majority of defects caused by NMDA receptor channel blockers were of structures derived from the neural tube and neural crest(Table 1). The drugs differed somewhat in the kinds of defect that they induced. For example, half of the defects induced by(+)MK-801 were spinal, whereas dextromethorphan induced spinal defects in only 3% of affected embryos; on the other hand, 40% of the defects induced by dextromethorphan were craniofacial. An example of cranial dysraphism induced by dextromethorphan is shown in the scanning electron photomicrographs of Figure 2.
Scanning electron photomicrographs of embryos harvested at 76 h. In each embryo the heart (H) has been broken away from the inferior aspect of the face for improved observation. In each case, the bar = 0.1 mm. (A) This embryo was treated with saline vehicle only and is normal. The epidermis is smooth and completely covers the dorsal aspect of the embryo (arrow). The eye (E) and the nasal placode (N) are placed normally. (B) This embryo was given 50 nmol/d (3 doses total) of dextromethorphan as described in “Methods.” A large neural tube dysraphism includes the region of the brain (B) and the spinal cord(S). The thin epidermis over the dysraphic neural tube is coarsely corrugated; this membrane is transparent for light microscopy, permitting visualization of the dysraphic neural tube. The small eye (E) is displaced caudally in relation to the nasal placode (N). This embryo was placed in the category, “multiple” defects (see Table 1). (C) This embryo was given 500 nmol/d dextromethorphan (3 doses total) as described in “Methods.” The thin epidermis was removed from the craniofacial region before the specimen was prepared for scanning electron microscopy to demonstrate the complete brain dysraphism (large arrow).
Glycine site antagonists. As a class, NMDA receptor antagonists that act at the glycine co-agonist site were less potent and efficacious in the induction of developmental defects using our model system(Fig. 3, top). However, 7-chlorokynurenic acid (500 nmol/embryo/d) elevated the incidence of neural tube lesions significantly(12.2 ± 3.1 defects/100 embryos, p < 0.01 versus control). Of the defects induced by 7-chlorokynurenic acid, 31% were spinal, 38% were craniofacial, and the remaining 31% were multiple site defects. The kynurenic acid derivatives increased mortality rates somewhat but the increase was not statistically significant (Fig. 3, bottom).
Effect of glycine site NMDA receptor antagonists on incidence of developmental defects and mortality in developing chicken embryos. During the first 2 d of development chicken embryos (groups of 18) were exposed to 5-500 nmol/embryo/d of various NMDA receptor glycine site antagonists as described in “Methods.” After 76-h development, embryos were examined for gross developmental abnormalities (top) or death(bottom). Data represent means ± SEM for defect and mortality rates in at least three separate experiments. Asterisks denote significance(**p < 0.01, vs vehicle control group). 7-Cl-Kyn, 7-chlorokynurenic acid; 7-Cl-Thiokyn, 7-chlorothiokynurenic acid.
Glutamate site antagonists. Glutamate site antagonists CPP and D-AP5 induced only a small increase in the occurrence of developmental defects(Fig. 4, top) and no increase in the mortality rate(Fig. 4, bottom). The L-isomer of D-AP5 (L-AP5, an NMDA receptor antagonist known to be less potent than D-AP5 in adult brain NMDA receptors) showed little activity.
Effect of glutamate site NMDA receptor antagonists on incidence of developmental defects and mortality in developing chicken embryos. During the first 2 d of development chicken embryos (groups of 18) were exposed to 5-500 nmol/embryo/d of various NMDA receptor channel blockers as described in “Methods.” After 76-h development, embryos were examined for gross developmental abnormalities (top) or death (bottom). Data represent means ± SEM for defect and mortality rates in at least three separate experiments.
Glutamate site agonists. Neither NMDA per se nor the highly potent NMDA receptor agonist HQ induced early developmental disorders at the doses tested (Fig. 4).
DISCUSSION
The results of this study demonstrate that exposure of early avian embryos to NMDA receptor antagonists, particularly NMDA receptor channel blockers, can result in the induction of neural crest/neural tube defects and can increase significantly the incidence of mortality in the early avian embryo. These data suggest a pivotal role for NMDA receptors, or perhaps another closely related receptor that is blocked by NMDA receptor antagonists, in neural tube development, neural crest cell migration, or differentiation. NMDA receptors mediate synaptic transmission and neural plasticity in the CNS(23–27). NMDA receptors are also known to regulate neuronal development by stabilizing converging synapses(28) by stimulating cerebellar granule cell migration, growth, and differentiation(29–33), and by modulating apoptosis(34–38).
NMDA receptors are comprised of at least two families of receptor subunits, NMDA-R1(39, 40) and NMDA-R2(41–43), both of which are required for a functional receptor. NMDA-R2 subunits represent four different gene products which impart most of the pharmacologic and functional diversity of NMDA receptors(42–44). Recent evidence has also shown that NMDA receptor subunits are differentially expressed during development(45, 46). The specific NMDA receptor subunits that are responsible for the teratogenic effects reported here are not shown by these experiments.
Knockout experiments with individual NMDA receptors failed to demonstrate a role for these receptors in neural tube development(47–50), suggesting the possibility that a closely related receptor that also is responsive to NMDA receptor antagonists [e.g. see Yuzaki et al.(51)] may be partly responsible for NMDA receptor teratogenicity, alternatively that receptor loss is somehow compensated for in the knockout mouse. There are other examples of such functional compensation: embryos exposed to neural cell adhesion molecule antibodies develop severe neural tube defects(52), whereas embryos that are homozygous-deficient for neural cell adhesion molecule do not(53).
Teratogens that induce neural tube developmental abnormalities include homocysteine(5), ethanol(12), and aliphatic hydrocarbons(13–15). Many of these compounds also have known NMDA receptor antagonist activity. We report here that exposure of avian embryos to NMDA receptor antagonists results in increases in neural tube developmental defects and embryonic mortality rates. The results of this study do not show why the NMDA receptor channel blocker class of compounds are the most potent and efficacious teratogens among the NMDA receptor antagonists; however, this efficacy may be due to differences in drug bioavailability. When 48-h incubated eggs were each given a single 50-nmol dose of (+)MK-801, dextromethorphan, homoquinolinic acid, or 5,7-dichlorokynurenic acid, each of which had been labeled with 3H, we observed concentrations of 24 ± 14, 67 ± 30, 2 ± 0.4, and 4 ± 2 μM, respectively (our unpublished observations, n = 3 embryos per drug). Hence, it appears that channel blocker antagonists MK-801 and dextromethorphan may concentrate to a much higher degree in the embryo than agonists or agonists of the glycine site (5,7-dichlorokynurenic acid) or glutamate site (homoquinolinic acid). This is consistent with the fact that channel blocker NMDA receptor antagonists tend to have chemical structures which lend to higher lipophilicity than the highly charged kynurenic acid or amino acid analogs.
The possibility that bioavailability per se may be a key feature in teratogenicity of the NMDA receptor antagonists is supported by our previous report on the teratogenicity of homocysteine(5). Although homocysteine may act as a glycine site antagonist of the NMDA receptor(6, 7), it is aggressively teratogenic(5). The resolution of this apparent ambiguity may be availability: like the calcium channel blockers but unlike the glycine site blockers used in the present study, homocysteine becomes highly concentrated in the embryos(5).
Another or alternative explanation for the difference in potency between the channel blocker class and glutamate site or glycine site antagonists may be in the physiology of NMDA receptor activation during development. If the developmental events that require activation of an NMDA receptor naturally involve a massive release of glutamate and glycine onto the active site, then the presence of relatively small amounts of competitive glutamate or glycine site antagonists would not be sufficient to overcome competition with these endogenous neurotransmitters. In contrast, a noncompetitive antagonist, by definition, would act independently of agonist concentration, and a noncompetitive or uncompetitive antagonist such as a channel blocker would be more effective.
Although these results were not derived directly from human or other mammalian embryos, a substantial amount of our present knowledge about heart and neural tube development has been acquired from studies of the avian embryo, and this knowledge has been shown to apply to mammals as well(54–56). Furthermore, modern techniques of molecular biology have shown clearly that the genes regulating early embryonic developmental events (e.g. neural tube closure and neural crest migration) are highly conserved. These include the genes whose perturbations are likely to be involved in the congenital defects reported here(e.g. PAX-3, sonic hedgehog, or brachyury)(57, 58). There are Drosophila, avian, murine, and human orthologs for each of these, therefore data derived from experiments with early avian embryos may perhaps be applied broadly to the expected results for other species when they are exposed to the same teratogens, including the early human embryo.
Thus, these results are alarming in light of the fact that the clinical use of various NMDA receptor antagonists is increasing. As a result of NMDA receptor involvement in neurodegenerative diseases, antagonists of NMDA receptors have recently emerged in clinical research(59) for the potential treatment of stroke, CNS trauma(60), epilepsy(61, 62), pain(63), Huntington's disease(64), AIDS dementia(65, 66); with possible application in Alzheimer's(67) and Parkinson's diseases(68, 69) as well. However, the most widespread use of an NMDA receptor antagonist, and perhaps the greatest potential threat to human embryos, is the nonprescription cough suppressant dextromethorphan, which is shown by the present data using this model to be a powerful inducer of both congenital defects and embryonic death when present in micromolar concentrations: a dose of 50 nmol/embryo/d caused significant embryo mortality in our experimental system. After a single 30-mg therapeutic dose, dextromethorphan in humans reaches micromolar concentrations in plasma(70), with a plasma half-life of 1-7 h(71). Furthermore, because there is a known polymorphic distribution in cytochrome P-450-dependent dextromethorphan metabolism(71), slow metabolizers of dextromethorphan would be expected to have more highly elevated and prolonged plasma levels after dextromethorphan ingestion. The results of the present study suggest that further studies of the teratogenic effect of dextromethorphan are warranted at this time.
Abbreviations
- MK-801:
-
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5,10-imine
- NMDA:
-
N-methyl-D-aspartate
- HQ:
-
homoquinolinic acid
- CPP:
-
DL-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid
- AP5:
-
D-2-amino-5-phosphopentanoic acid
References
Serbedzija GN, Bronner Fraser M, Fraser SE 1994 Developmental potential of trunk neural crest cells in the mouse. Development 120: 1709–1718
Nickel RE, Pillers DA, Merkens M, Magenis RE, Driscoll DA, Emanuel BS, Zonana J 1994 Velo-cardio-facial syndrome and DiGeorge sequence with meningomyelocele and deletions of the 22q11 region. Am J Med Genet 52: 445–449
Guttormsen AB, Schneede J, Ueland PM, Refsum H 1996 Kinetics of total plasma homocysteine in subjects with hyperhomocysteinemia due to folate or cobalamin deficiency. Am J Clin Nutr 63: 194–202
Stabler SP, Lindenbaum J, Allen RH 1996 The use of homocysteine and other metabolites in the specific diagnosis of vitamin B-12 deficiency. J Nutr 126: 1266s–1272s
Rosenquist TH, Ratashak SA, Selhub J 1996 Homocysteine induces congenital defects of the heart and neural tube: effect of folic acid. Proc Natl Acad Sci USA 93: 15227–15232
Kim WK, Rayadu PV, Choi YB, Kumar S, Stamler JS, Lipton SA 1995 Homocysteine: an NMDA agonist and glycine site partial agonist that induces cortical neurotoxicity. Soc Neurosci Abstr 1995
Kim WK, Pae YS 1996 Involvement ofN-methyl-D-aspartate receptor and free radical in homocysteine-mediated toxicity on rat cerebellar granule cells in culture. Neurosci Lett 216: 117–120
Lovinger DM, White G, Weight FF 1989 Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724
Grant KA, Lovinger DM 1995 Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci 3: 155–164
Hoffman PL, Tabakoff B 1996 Alcohol dependence: a commentary on mechanisms. Alcohol 31: 333–340
Bhave SV, Snell LD, Tabakoff B, Hoffman PL 1996 Mechanism of ethanol inhibition of NMDA receptor function in primary cultures of cerebral cortical cells. Alcohol Clin Exp Res 20: 934–941
Tsai G, Gastfriend DR, Coyle JT 1995 The glutamatergic basis of human alcoholism [see comments]. Am J Psychiatry 152: 332–340
Bove FJ 1996 Public drinking water contamination and birthweight, prematurity, fetal deaths, and birth defects. Toxicol Ind Health 12: 255–266
Bove FJ, Fulcomer MC, Klotz JB, Esmart J, Dufficy EM, Savrin JE 1995 Public drinking water contamination and birth outcomes [see comments]. Am J Epidemiol 141: 850–862
Elovaara E, Hemminki K, Vainio H 1979 Effects of methylene chloride, trichloroethane, trichloroethylene, tetrachloroethylene and toluene on the development of chick embryos. Toxicology 12: 111–119
Martin DC, Abraham JE, Plagenhoef M, Aronstan RS 1991 Volatile anesthetics and NMDA receptors. Enflurane inhibition of glutamate-stimulated [3H]MK-801 binding and reversal by glycine. Neurosci Lett 132: 73–76
Aronstam RS, Martin DC, Dennison RL 1994 Volatile anesthetics inhibit NMDA-stimulated 45Ca uptake by rat brain microvesicles. Neurochem Res 19: 1515–1520
Martin DC, Aronstam RS 1995 Spermidine attenuation of volatile anesthetic inhibition of glutamate-stimulated[3H](5R,10S)-(+)-methyl-10,11-dihydro-5H- dibenzo-[a,d]cyclohepten-5,10-imine ([3H]MK-801) binding to N-methyl-D-aspartate (NMDA) receptors in rat brain. Biochem Pharmacol 50: 1373–1377
Martin DC, Plagenhoef M, Abraham J, Dennison RL, Aronstam RS 1995 Volatile anesthetics and glutamate activation of N-methyl-D-aspartate receptors. Biochem Pharmacol 49: 809–817
Daniell LC 1994 Effect of anesthetic and convulsant barbiturates on N-methyl-D-aspartate receptor-mediated calcium flux in brain membrane vesicles. Pharmacology 49: 296–307
Choi DW, Peters S, Viseskul V 1987 Dextrorphan and levorphanol selectively block N-methyl-D-aspartate receptor-mediated neurotoxicity on cortical neurons. J Pharmacol Exp Ther 242: 713–720
Ferkany JW, Borosky SA, Clissold DB, Pontecorvo MJ 1988 Dextromethorphan inhibits NMDA-induced convulsions. Eur J Pharmacol 151: 151–154
Monaghan DT, Bridges RJ, Cotman CW 1989 The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol 29: 365–402
Collingridge GL, Lester RA 1989 Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev 41: 143–210
McBain CJ, Mayer ML 1994 N-Methyl-D-aspartic acid receptor structure and function. Physiol Rev 74: 723–760
Monaghan DT, Buller AL, Andaloro VJ 1996 On the molecular basis of NMDA receptor diversity. In: Ionotropic Glutamate Receptors. Humana Press, Tottowa, NJ
Sucher NJ, Awobuluyi M, Choi YB, Lipton SA 1996 NMDA receptors: from genes to channels. Trends Pharmacol Sci 17: 348–355
Scheetz AJ, Constantine Paton M 1994 Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB J 8: 745–752
Cambray Deakin MA, Foster AC, Burgoyne RD 1990 The expression of excitatory amino acid binding sites during neuritogenesis in the developing rat cerebellum. Brain Res Dev Brain Res 54: 265–271
Burgoyne RD, Graham ME, Cambray Deakin M 1993 Neurotrophic effects of NMDA receptor activation on developing cerebellar granule cells. J Neurocytol 22: 689–695
Farrant M, Feldmeyer D, Takahashi T, Cull Candy SG 1994 NMDA-receptor channel diversity in the developing cerebellum. Nature 368: 335–339
Komuro H, Rakic P 1993 Modulation of neuronal migration by NMDA receptors. Science 260: 95–97
Rossi DJ, Slater NT 1993 The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology 32: 1239–1248
Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P 1995 Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15: 961–973
Ankarcrona M, Zhivotovsky B, Holmstrom T, Diana A, Eriksson JE, Orrenius S, Nicotera P 1996 Lamin and β-tubulin fragmentation precede chromatin degradation in glutamate-induced neuronal apoptosis. Neuroreport 7: 2659–2664
Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA 1995 Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA 92: 7162–7166
Finiels F, Robert JJ, Samolyk ML, Privat A, Mallet J, Revah F 1995 Induction of neuronal apoptosis by excitotoxins associated with long-lasting increase of 12-O-tetradecanoylphorbol 13-acetate-responsive element-binding activity. J Neurochem 65: 1027–1034
McDonald JW, Johnston MV 1993 Excitatory amino acid neurotoxicity in the developing brain. NIDA Res Monogr 133: 185–205
Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S 1991 Molecular cloning and characterization of the rat NMDA receptor [see comments]. Nature 354: 31–37
Yamazaki M, Mori H, Araki K, Mori KJ, Mishina M 1992 Cloning, expression and modulation of a mouse NMDA receptor subunit. FEBS Lett 300: 39–45
Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M 1992 Molecular diversity of the NMDA receptor channel [see comments]. Nature 358: 36–41
Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH 1992 Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256: 1217–1221
Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S 1993 Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem 268: 2836–2843
Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH 1994 NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron 12: 1031–1040
Watanabe M, Inoue Y, Sakimura K, Mishina M 1992 Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 3: 1138–1140
Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY 1994 Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368: 144–147
Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T 1994 Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron 13: 325–338
Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S 1994 Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell 76: 427–437
Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M 1996 Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron 16: 333–344
Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M 1995 Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature 373: 151–155
Yuzaki M, Forrest D, Curran T, Connor JA 1996 Selective activation of calcium permeability by aspartate in Purkinje cells. Science 273: 1112–1114
Bronner Fraser M, Wolf JJ, Murray BA 1992 Effects of antibodies against N-cadherin and N-CAM on the cranial neural crest and neural tube. Dev Biol 153: 291–301
Cremer H, Chazal G, Goridis C, Represa A 1997 NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci 8: 323–335
Kirby ML, Gale TF, Stewart DE 1983 Neural crest cells contribute to normal aorticopulmonary septation. Science 220: 1059–1061
Kirby ML, Turnage KLd, Hays BM 1985 Characterization of conotruncal malformations following ablation of cardiac neural crest. Anat Rec 213: 87–93
Kirby ML, Waldo KL 1990 Role of neural crest in congenital heart disease. Circulation 82: 332–340
Holland PW, Graham A 1995 Evolution of regional identity in the vertebrate nervous system. Perspect Dev Neurobiol 3: 17–27
Witt G, Rosenquist TH, Gadson PF 1997 Homocysteine inhibition of PAX-3 expression. FASEB J 11:A427.
Lipton SA 1993 Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci 16: 527–532
Faden AI, Salzman S 1992 Pharmacological strategies in CNS trauma. Trends Pharmacol Sci 13: 29–35
Perucca E 1993 The clinical pharmacology of the new antiepileptic drugs. Pharmacol Res 28: 89–106
Thomas RJ 1995 Excitatory amino acids in health and disease. J Am Geriatr Soc 43: 1279–1289
Elliott K, Kest B, Man A, Kao B, Inturrisi CE 1995 N-methyl-D-aspartate (NMDA) receptors, mu and kappa opioid tolerance, and perspectives on new analgesic drug development. Neuropsychopharmacology 13: 347–356
Purdon SE, Mohr E, Ilivitsky V, Jones BD 1994 Huntington's disease: pathogenesis, diagnosis and treatment. J Psychiatry Neurosci 19: 359–367
Lipton SA 1992 Models of neuronal injury in AIDS: another role for the NMDA receptor? [see comments]. Trends Neurosci 15: 75–79
Lipton SA 1994 HIV-related neuronal injury. Potential therapeutic intervention with calcium channel antagonists and NMDA antagonists. Mol Neurobiol 8: 181–196
Barry SR 1991 Clinical implications of basic neuroscience research. IL NMDA receptors and neurotrophic factors. Arch Phys Med Rehabil 72: 1095–1101
Ossowska K 1994 The role of excitatory amino acids in experimental models of Parkinson's disease. J Neural Transm Parkinson's Dis Dementia Sect 8: 39–71
Rogawski MA 1993 Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2:3-benzodiazepines. Trends Pharmacol Sci 14: 325–331
Kazis A, Kimiskidis V, Niopas I 1996 Pharmacokinetics of dextromethorphan and dextrorphan in epileptic patients. Acta Neurol Scand 93: 94–98
Nagai N, Kawakubo T, Kaneko F, Ishii M, Shinohara R, Saito Y, Shimamura H, Ohnishi A, Ogata H 1996 Pharmacokinetics and polymorphic oxidation of dextromethorphan in a Japanese population. Biopharm Drug Dispos 17: 421–433
Acknowledgements
The authors gratefully acknowledge the technical assistance of S. Anne Ratashak. Some of these experiments were carried out with the assistance of Annette Bojanski and Sheldon Thieszen during the research elective of their senior year in medical school.
Author information
Authors and Affiliations
Additional information
Supported by U.S. Public Health Service Grant RO1-HL-55940.
Rights and permissions
About this article
Cite this article
Andaloro, V., Monaghan, D. & Rosenquist, T. Dextromethorphan and Other N-Methyl-D-Aspartate Receptor Antagonists Are Teratogenic in the Avian Embryo Model. Pediatr Res 43, 1–7 (1998). https://doi.org/10.1203/00006450-199801000-00001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199801000-00001
This article is cited by
-
Glutamatergic neurotransmission in alcoholism
Journal of Biomedical Science (1998)