Abstract
Before puberty, the diagnosis of androgen insensitivity syndrome (AIS) can be difficult. We studied whether the decrease of sex hormone-binding globulin(SHBG) during the human chorionic gonadotropin (hCG) test may represent a biochemical test to select prepubertal patients with AIS. We examined prepubertal patients with AIS (n = 9, age 0.9-8.2 y), male pseudohermaphroditism not due to AIS (other-MPH) (n = 8, age 0.6-10.7 y), and control boys (n = 12, age 0.8-12.5 y). Testosterone and SHBG levels (mean ± SD) were measured before (d 0) and after (d 5) a hCG test (1500 IU × 3 d). Testosterone levels (nmol/L) increased in all groups [AIS: from 1.5 ± 1.2 to 22.1 ± 11.8 (p < 0.001); other-MPH: from 0.6 ± 0.6 to 9.2 ± 7.4 (p < 0.02); controls: from 1.8 ± 1.4 to 22.8 ± 14.4 (p < 0.001)]. SHBG concentrations (nmol/L) did not change in AIS [from 66.2± 15.1 to 67.5 ± 18.6 (p = NS), Δ-variation 1.7± 12.7%], whereas they were significantly decreased in other-MPH [from 59.9 ± 14.2 to 46.5 ± 18.6 (p < 0.005),Δ-variation -23.7 ± 19.6%] and controls [from 63.0 ± 16.9 to 33.7 ± 14.6 (p < 0.003), Δ-variation -46.9± 15.2%]. Our data suggest that the SHBG changes during the hCG test can be used to assess in vivo the biologic response to androgens in prepubertal patients with ambiguous genitalia, selecting those patients in whom it is worth performing second level investigations to confirm the AIS diagnosis.
Similar content being viewed by others
Main
AIS represents a main cause of MPH(1, 2). It is an X-linked disorder that results from genetic mutations in the androgen receptor gene(1). The defective androgen receptor shows an absent or impaired response to androgens in target tissues, although testosterone synthesis in affected individuals is unimpaired(1, 2). AIS patients have a normal 46,XY karyotype and well differentiated testes, but a spectrum of defects in male sexual development: clinical phenotypes range from normal females to normal infertile males(1, 2).
Although postpubertal individuals with AIS have a typical endocrine patterni.e. elevated serum concentrations of LH with elevated or high-normal serum levels of testosterone and estrogens(1, 2), before puberty the differential diagnosis of AIS from other forms of MPH can be extremely difficult, because the hormonal findings are not specific(3–7). In a series of 15 patients with suspected AIS on the basis of clinical and endocrine findings, referred to us to establish the diagnosis by studying the binding of androgen receptors in genital skin fibroblasts or molecular genetics of the androgen receptor gene(1), only 4 (26.6%) had the diagnosis proved by these techniques(8). Because both techniques are time-consuming and affected by high cost and complexity, the patients with suspected AIS should be carefully selected by clinical examination and endocrinologic tests before these second level investigations are performed.
These considerations stimulated the search for simple clinical tests to assess in vivo androgen sensitivity in prepubertal patients with MPH. Some authors proposed the stimulation of nitrogen retention after androgen administration to investigate androgen sensitivity in a clinical setting(9). However, this test is not simple and requires a strict control of nitrogen balance for several days in hospitalized patients. As androgens decrease the serum levels of the SHBG(10), it has been proposed that a measure of SHBG variations under androgen stimulation may be used as a clinical test of androgen sensitivity, but conclusive data have not been been found in prepubertal patients(11, 12).
We studied the variations of SHBG during hCG tests in prepubertal patients with proved AIS or with other causes of MPH and in normal boys to investigate whether SHBG changes may discriminate AIS patients from the other boys.
METHODS
Patients. We examined nine prepubertal patients with demonstrated AIS (complete AIS, n = 4; partial AIS, n = 5; ages 0.9-8.2 y) (Table 1); AIS was diagnosed by karyotype analysis, clinical phenotype, family history, altered binding of androgen receptors in genital skin fibroblasts, and/or by identification of mutations in the androgen receptor gene (Table 1).
We also examined eight prepubertal patients with MPH not due to AIS(other-MPH) (ages 0.6-10.7 y) (Table 1) and 12 normal prepubertal boys (ages 0.8-12.5 y) as controls. Control boys were sons of medical or technical staff of our departments; they had normal genitalia at clinical examination and a family history excluding any disease related to the male reproductive tract.
Because SHBG levels in prepubertal children is affected by weight(13), relative body weight was calculated in each subject (patients and controls). Before or during the study, no subjects received any drugs that may alter SHBG serum levels(10).
Informed consent was obtained from both the parents of each subject(patients and controls), and the study was approved by the ethical committees of our departments.
Procedures. The relative body weight was expressed as a percentage of the normal mean weight of children of the same height and sex. Normative auxologic values were derived from Tanner's tables(14). In patients, normal values were chosen according to assigned sex.
In each patient and control subject, serum levels of testosterone and SHBG concentrations were measured before and after the hCG test. The hCG test was performed as follows. hCG (Lab. Serono, Switzerland), 1500 IU/daily for 3 consecutively days, was administered by intramuscular injection. Blood specimens for testosterone and SHBG determinations were collected in each subject after an overnight fast before and 4 d after the first hCG injection. All injections were made in the morning (0800-0900) by a nurse trained in pediatric endocrinology; and the blood samples were centrifuged at 4°C within 1 h from withdrawal, aliquoted in 1.0-mL serum fractions, and stored at-20°C until assayed.
Serum levels of testosterone and SHBG were measured by commercially available kits (DPC, Los Angeles, CA; Spectria, Orion Diagnostica, Finland, respectively). In our laboratory, testosterone inter- and intraassay coefficients of variation were 8.7 and 7.2%, respectively; SHBG inter- and intraassay coefficients of variation were 4.8 and 3.5%, respectively. Each set of samples from the same patient was run in the same assay.
Genital skin fibroblasts were obtained by skin biopsy and grown in 90-mm Petri dishes in Dulbecco's modified Eagle's medium (Sigma Chemical Co., Italy) supplemented with L-glutamine, antibiotics/antimycotic (Sigma), and 15% fetal bovine serum (Sigma). Specific binding of [3H]dihydrotestosterone(Amersham Corp., UK) to the androgen receptors of skin fibroblasts after incubation at 37°C for 1 h was performed as described by Gad et al.(15). The maximum binding capacity(Bmax) and the apparent dissociation constant(Kd) of the androgen receptors were determined by Scatchard plots(16).
PCR and molecular screening of the androgen receptor gene were performed according to Brown et al.(17). Briefly, 10-20 mL of venous blood were collected from each patient, and genomic DNA was purified by standard techniques. PCR primer sequences were those published by Macke et al.(18). Seven overlapping fragments of approximately 250 bp were used to amplify the first exon of androgen receptor gene. Exons 2-8, together with at least 30 bp of flanking intron sequence, were amplified as a single PCR fragment. In each reaction one primer carried a 40-bp GC-rich clamp at the 5′ end to facilitate detection of mutation by DGGE(19). To increase the sensitivity of DGGE, heteroduplexes that migrate more slowly than homoduplexes were formed between pairs of samples by an additional cycle of denaturation and renaturation(18). PCR products were resolved by DGGE according to the method of Brown et al.(17). The amplifications that produced a variant pattern on DGGE were sequenced by using the dideoxy-chain termination method(20) with a commercial kit (Silver Sequence, Promega, Madison, WI).
Cytogenetic analysis was performed on peripheral blood leukocytes, and karyotypes were determined by examining 40-50 metaphases from each patient by standard techniques.
Statistical analyses. Results are expressed as mean ± SD. The changes in SHBG serum levels during hCG tests are expressed as percent variations (Δ%) versus the baseline values according to the formula: (value after - value before/value before) × 100. The differences between the groups were assessed by unpaired Wilcoxon's(Mann-Withney) rank-sum test using a statistical system (LabStat.303, SIBIOC, Milan) adapted for an IBM personal computer. The significance of changes in each group after the hCG test was analyzed by using the paired t test or by Wilcoxan signed rank test for pairs, where appropriate. In all statistical analyses, p < 0.05 was considered significant.
RESULTS
Testosterone. Before hCG stimulation, serum testosterone levels(nmol/L) were not significantly different in the three groups of subjects [AIS 1.5 ± 1.2 (0.4 ± 0.3 ng/mL); other-MPH 0.6 ± 0.6 (0.2± 0.2 ng/mL) (p = NS versus AIS and controls); controls 1.8 ± 1.4 (0.5 ± 0.4 ng/mL) (p = NSversus AIS and other-MPH)].
During the hCG test, testosterone levels significantly increased in AIS patients and in controls [testosterone peak, nmol/L: AIS 22.1 ± 11.8(6.4 ± 3.4 ng/mL) (p < 0.001 versus baseline); controls 22.8 ± 14.4 (6.6 ± 4.1 ng/mL) (p < 0.0005versus baseline)] (Fig. 1). No significant difference in testosterone peak was found between complete AIS [testosterone peak, nmol/L: 22.5 ± 15.2 (6.5 ± 4.4 ng/mL)] and partial AIS(testosterone peak, nmol/L: 21.9 ± 10.2 (6.3 ± 2.9 ng/mL)] and between controls and AIS. As a group, mean testosterone peak [9.2 ± 7.4 nmol/L (2.6 ± 2.1 ng/mL); p < 0.002 versus baseline] in the other-MPH patients was significantly reduced (p< 0.005) in respect to that of AIS and controls. At any rate, the testosterone response to hCG in other-MPH cases varied widely according to the individual diagnosis; for example, in the patients with congenital Leydig cell hypoplasia, no effect of hCG administration was observed on testosterone levels, whereas in the remaining subjects a variable testosterone rise was found (Fig. 1).
Individual variations of serum testosterone levels(nmol/L) during the hCG test. In the middle panel, the broken lines represent the patients with complete AIS and the solid lines those with partial AIS. In the lower panel, the broken lines represent the patients with congenital Leydig cell hypoplasia and the solid lines those with other forms of MPH (see Table 1).
SHBG. Before hCG stimulation, serum SHBG levels (nmol/L) were not significantly different in the three groups of subjects [AIS 66.2 ± 15.1 (19.3 ± 4.4 ng/mL), other-MPH 59.9 ± 14.2 (17.5 ± 4.1 ng/mL) (p = NS versus AIS and controls); controls 63.0± 16.9 (18.4 ± 4.9 ng/mL) (p = NS versus AIS and other-MPH)].
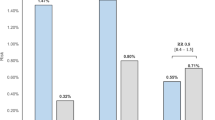
During the hCG test, serum SHBG levels showed a significant decrease in control subjects [after hCG values 33.7 ± 14.6 nmol/L (9.8 ± 4.2 ng/mL); p < 0.003 versus baseline]. The meanΔ-variation of SHBG was -46.9 ± 15.2%. Therefore, aΔ-variation of at least - 16.5% was adopted as a cut-off value (2 SD) to define a positive response to hCG test (Fig. 2).
Individual variations of serum SHBG levels(Δ-variation%) during the hCG test. In the middle panel, the broken lines represent the patients with complete AIS and the solid lines those with partial AIS; the horizontal dotted line represents the minimalΔ-variation% in controls that define a positive response. In the lower panel, the broken lines represent the patients with congenital Leydig cell hypoplasia and the solid lines those with other forms of MPH (see Table 1); the horizontal dotted line represents the minimal Δ-variation% in controls that define a positive response.
SHBG concentrations did not significantly change in AIS subjects [mean after hCG value 67.5 ± 18.6 nmol/L (19.7 ± 5.4 ng/mL);p = NS versus baseline); mean Δ-variation of SHBG in this group was 1.7 ± 12.7%. No significant differences were found between complete AIS (Δ-variation 0.1 ± 14.7%) and partial AIS(Δ-variation 3.0 ± 12.5%, p = NS versus complete AIS). No overlap in Δ-variations of SHBG among controls and AIS patients was found (Fig. 2).
Other-MPH patients, showed a variable SHBG response to hCG test. In Leydig cell hypoplasia, SHBG remained substantially unchanged [case 10, d 0: 88.1 nmol/L (25.7 ng/mL), d 5: 84.0 nmol/L (24.5 ng/mL), Δ-variation -4.6%; case 11, d 0: 53.5 nmol/L (15.6 ng/mL), d 5: 58.6 nmol/L (17.1 ng/mL),Δ-variation 9.5%)], whereas in the remaining MPH patients (n = 6), SHBG decreased significantly [after hCG: 38.7 ± 12.2 nmol/L (11.3± 3.5 ng/mL); p < 0.005 versus baseline). MeanΔ-variation was less than in control group (-31.8 ± 13.9%,p < 0.05 versus controls) and some overlap with AIS patients was observed in individual subjects (Fig. 2). Nevertheless, as a group, mean Δ-variation was significantly larger in other-MPH as compared with AIS patients (p < 0.002).
Relative body weight was not significantly different in the three groups of subjects [AIS: 99.6 ± 10.9% (range 83-114%); controls: 103.8 ± 10.9% (range 85-123%); other-MPH 98.0 ± 11.9% (range 81-119%)].
DISCUSSION
AIS is the most common cause of MPH(1, 2). This syndrome is due to a diminished or absent androgen action in target tissues for genetic alterations of the androgen receptor(1, 2), as we confirm in the partial AIS patients of our series, in whom point mutations in the hormone binding domain of the androgen receptor gene were detected(1). All but two of point mutations we found have been previously reported in partial AIS subjects [see Quigley et al.(1) for review]. Such mutations were able to alter functional activity of androgen receptor(1). The mutation of patient 5 was not previously described, but a 3-bp deletion at position 690 in exon 4 has been observed in partial AIS patients of a single family(21). To our knowledge, the mutation of patient 6 was not described. We have not yet performed transfection studies, but the clinical data of the patient together with the finding that several point mutations close to those we found are associated with partial AIS(1) strongly suggest that the genetic alteration was the cause of clinical phenotype.
In prepubertal boys, endocrinologic diagnosis of AIS is usually very difficult(1–7). In our as well as in other studies(4, 6, 22), baseline or stimulated testosterone levels did not differentiate patients with partial or complete AIS from healthy boys or from some forms of other-MPH. On the contrary, other authors stated that they found increased baseline levels of testosterone in complete AIS patients after 6 mo of age [unpublished results cited in Grumbach and Conte(2)]. Before the 6th mo, the physiologic surge of LH and testosterone that occurs in normal male infant(2) has not been detected in complete AIS patients[unpublished results of C. A. Quigley and F. S. French cited in Quigley et al.(1), and unpublished results of J.-L. Chaussain, cited in Grumbach and Conte(2)], whereas other authors reported increased basal or stimulated levels of testosterone(22, 23). Regarding partial AIS, basal and stimulated testosterone levels have been found increased in the few infants in whom they have been measured(5, 7, 24). Thus, basal and stimulated testosterone levels can be useful in recognizing AIS patients, before 6th mo of life, when the pituitary-gonadal axis is physiologically activated(2), but the small number of reported patients preclude definitive conclusions.
Even clinical tests, as the increase in penile length in response to the rise of testosterone levels after its direct injection or secondary to hCG administration(25, 26), have been judged to be not sensitive enough for selecting patients with AIS(6, 7). In fact, phallic growth showed large variations also in patients with idiopathic micropenis [direct testosterone test: from +0.9 to +3.2 cm; hCG test: from +0.25 to +0.75 cm (12.5-50% of baseline length)](25, 26); furthermore, some phallic increase has been found also in patients with AIS(3, 6). In addition, exact measurement of penile length, mainly in the case of micropenis or clitoromegaly, may be difficult to perform.
Because clinical and endocrine findings are not conclusive in prepubertal boys with ambiguous genitalia and 46,XY karyotype, AIS is usually diagnosed by androgen receptor binding assay in cultured genital skin fibroblasts or by molecular genetic analysis of the androgen receptor gene(1, 2). At best, both these methods are available only in a small number of laboratories, require high costs, and, usually, the results are available only after several weeks. Thus, they are not reliable for all suspected patients, and clinical or biochemical tests are needed to select those cases who require such second level techniques.
Because SHBG is sensitive to androgens(10) and its serum levels can be accurately measured by simple laboratory methods, Belgoroski and Rivarola(11, 12) proposed to use the testosterone-induced SHBG decrease during the hCG test or direct testosterone administration to test androgen sensitivity in the clinical setting. These authors concluded that, although the lack of the SHBG decrease after exogenous testosterone may be a useful test for the selection of patients with AIS from those with other forms of MPH, variation of SHBG after the hCG test may not be(12). In some cases, a combination of a hCG test plus testosterone administration could be helpful(12). Moreover, all but one of the prepubertal patients studied by Belgoroski and Rivarola(12) had not a proved AIS diagnosis, although proved AIS subjects were not investigated by hCG test alone(12). In addition, the control group used by these authors was cryptorchid boys(11) selected on a clinical basis; a subtle impairment of hypophyseal-testicular axis not evident on clinical examination may be present in some of these boys, impairing SHBG response. In contrast, we studied prepubertal patients with a well established AIS diagnosis by reference methods(1, 15, 17) together with a control group of prepubertal healthy boys without any alterations of their genitalia. Although hCG induced a comparable rise in serum testosterone in AIS and in control subjects, SHBG levels significantly decreased in controls but not in AIS patients. No significant differences in SHBG levels were found between complete or partial AIS. We do not have a clear explanation for this result, but the low number of subjects in each of the two subgroups may be the reason. Additional patients should be studied to shed light on this matter.
In other-MPH patients, the mean SHBG levels after the hCG test was significantly lower than in AIS subjects, but significantly higher than in controls. Nevertheless, individual data analysis showed that some overlap among the three groups was present. It may be due to the variable increase in testosterone values during the hCG test in the other-MPH patients. For example, in patients with congenital Leydig cell hypoplasia, who had no testosterone rise, no SHBG variation was observed, as in AIS. In the other-MPH subjects with poor SHBG response, other clinical data (history, clinical examination, karyotype, sonography, testosterone response to hCG test) may provide the right diagnosis.
The other important regulator of SHBG levels in children, i.e. weight(10, 13), is likely not involved in our results, because SHBG baseline values as well as relative body weights are not different among the three studied groups. Moreover, large weight variations cannot occur in the few days of the test, and no patient received any drugs that interfered with SHBG serum levels.
The testosterone secretion test is mandatory in patients with MPH(2). Our data confirm the original Belgoroski and Rivarola(11) observation that measurement of both testosterone and SHBG during hCG test permit the study of peripheral androgen sensitivity in addition to Leyding cell function in boys with sex disorders. If our and other data(11, 12) will be confirmed in younger infants, this test might allow a better management of patients with ambiguous genitalia, particularly in selecting infants for molecular genetic studies of androgen receptor gene and in defining the sex of rearing(11). At any rate, the efficacy of this test in pubertal subjects or in infants during the first 6 mo of life should be assessed, because previous studies reported that the test is of limited use in subjects with active testicular secretion [i.e. testosterone values greater than 3.47 nmol/L (1.0 ng/mL)](12).
In conclusion, our data suggest that the changes of SHBG concentrations during the hCG test could be used as a screening test to assess in vivo the biologic response to androgens in prepubertal males, permitting the selection of patients who require second level investigations (steroid binding assay, molecular genetics) to confirm the diagnosis of AIS.
Abbreviations
- AIS:
-
androgen insensitivity syndrome
- DGGE:
-
denaturing gradient-gel electrophoresis
- hCG:
-
human chorionic gonadotropin
- MPH:
-
male pseudohermaphroditism
- PCR:
-
polymerase chain reaction
- SHBG:
-
sex hormone-binding globulin
References
Quigley CA, De Bellis A, Marschke KB, El-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16: 271–321
Grumbach MM, Conte FA 1992 Disorders of sex differentiation. In: Wilson JD, Foster DW (eds) Willims' Textbook of Endocrinology, 8th Ed., WB Saunders, Philadelphia, pp 853–951
Maes M, Lee PA, Jeffs RD, Sultan C, Migeon CJ 1980 Phenotypic variations in a family with partial androgen insensitivity syndrome. Am J Dis Child 134: 470–473
Berkovitz JD, Lee PA, Brown TR, Migeon CJ 1984 Etiologic evaluation of male pseudohermaphroditism in infancy and childhood. Am J Dis Child 138: 755–759
Lee PA, Brown TR, LaTorre HA 1986 Diagnosis of partial androgen insensitivity syndrome during infancy. JAMA 255: 2207–2209
Forest MG, Mollard P, David M, Morel Y, Bertrand J 1990 Syndrome d'insensibilitè incompléte aux androgènes. Difficultés du diagnostic et la conduite à tenir. Arch Fr Pediatr 47: 107–113
Saggese G, Battini R, Bertelloni S 1994 Diagnosi precoce di insensibilità agli androgeni. Riv Ital Pediatr 20: 28–36
Battini R, Bertelloni S, Federico G, Brown TR, Saggese G 1993 Endocrinological, receptorial and molecular evaluation of male pseudohermaphroditism. J Endocrinol Invest 16( suppl 1): 5.
Zachmann M, Zagalak M, Vollmin JA, Gitzelmann RP, Prader A 1977 Influence of testosterone on urinary 15N-balance in normal subjects and in patients with testicular feminization. Clin Chim Acta 77: 147–152
Rosner W 1991 Plasma steroid-binding proteins. Endocrinol Metab Clin North Am 20: 697–719
Belgoroski A, Rivarola MA 1982 Sex hormone-binding globulin response to human chorionic gonadotropin stimulation in children with cryptorchidism, anorchia, male pseudohermaphroditism, and micropenis. J Clin Endocrinol Metab 54: 698–704
Belgoroski A, Rivarola MA 1985 Sex hormone-binding globulin response to testosterone. An androgen sensitivity test. Acta Endocrinol 109: 130–138
Dunkel L, Sorva R, Voutilainen R 1985 Low levels of sex hormone-binding globulin in obese children. J Pediatr 107: 95–97
Tanner JM, Whitehouse RH, Takaishi M. 1966 Standards from birth to maturity for height, weight, height velocity and weight velocity: British children-1965. Arch Dis Child. 41: 613–634.
Gad YZ, Berkovitz GD, Migeon CJ, Brown TR 1988 Studies of up-regulation of receptors in genital skin fibroblasts. Mol Cell Endocrinol 57: 205–213
Scatchard G 1949 The attraction of proteins for small molecules and ions. Ann NY Acad Sci 51: 660–672
Brown TR, Lubahn DB, Wilson EM, French FS, Migeon CJ, Corden JL 1990 Functional characterization of naturally occurring androgen receptors from patients with complete androgen insensitivity. Mol Endocrinol 4: 1759–1772
Macke JP, Hu N, Hu S, Bailey M, King VL, Brown T, Hamer D, Nathans J 1993 Sequence variation in androgen receptor gene is not a common determinant of male sexual orientation. Am J Hum Genet 53: 844–852
Sheffield VC, Cox DR, Lerman LS, Myers RM 1989 Attachment of a GC-clamp to genomic DNA fragments by the polymerase chain reaction results in improved detection of single base changes. Proc Natl Acad Sci USA 86: 232–236
Sanger F, Nicklen S, Coulson AR 1977 DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Schwartz M, Skovby F, Muller J, Nielsen O, Skakkebaek NE 1994 Partial androgen insensitivity (PAIS) in a large Eskimo kindred caused by a DD690 mutation in the androgen receptor gene. Horm Res 41: 117(abstr 244)
Forest MG 1984 La réponse stéroidogénique testiculaire à la gonadotrophine chorionique est augmentée pedant le premiere trimestre de la vie dans le syndrome de féminisation testiculaire. Colloq INSERM 123: 341–346
van Zijl JAWM, Evers JLH, Gerver WJM 1990 Testicular feminization in the neonate. Gynecol Obstet Invest 29: 161–164
Nagel RA, Lippe BM, Griffin JE 1986 Androgen resistance in the neonate: use of hypothalamic-pituitary-gonadal axis for diagnosis. J Pediatr 109: 486–488
Burstein S, Grumbach MM, Kaplan S 1979 Early determination of androgenresponsiveness is important in the management of microphallus. Lancet 2: 983–986
Almaguer MC, Saenger P, Linder BL 1993 Phallic growth after hCG. A clinical index of androgen responsiveness. Clin Pediatr 32: 329–333
Acknowledgements
The authors acknowledge the children and their parents who made this work feasible; we also appreciate the assistance of Patrizia Gerini in the management of the patients. We are indebted to Dr. T. R. Brown (Johns Hopkins University, School of Medicine, Baltimore) for his helpful suggestions, and are grateful to Dr. P. Simi (U.O. Citogenetica, Azienda Ospedaliera Pisana, Pisa, Italy) for karyotyping some of the patients.
Author information
Authors and Affiliations
Additional information
Supported in part by MURST (Fondi 40%), Rome, Italy.
Rights and permissions
About this article
Cite this article
Bertelloni, S., Federico, G., Baroncelli, G. et al. Biochemical Selection of Prepubertal Patients with Androgen Insensitivity Syndrome by Sex Hormone-Binding Globulin Response to the Human Chorionic Gonadotropin Test. Pediatr Res 41, 266–271 (1997). https://doi.org/10.1203/00006450-199702000-00018
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199702000-00018
This article is cited by
-
Management of disorders of sex development
Nature Reviews Endocrinology (2014)