Abstract
Because inflammation could affect lysosomal enzyme trafficking, resulting in increased enzyme release from the cells, tissue necrosis, or altered blood- and the brain-cerebrospinal fluid (CSF) barrier, the activity of four lysosomal enzymes in the cell-free CSF of 34 patients with bacterial meningitis, 20 with aseptic meningitis, and 39 control subjects was measured. Activities are expressed in nanomoles of 4-methylumbelliferone/mL/h. The median β-hexosaminidase A activity in bacterial meningitis was 313, in aseptic meningitis it was 173, and in the control subjects it was 175; the median β-hexosaminidase B activity was 417, 165, and 120; the medianα-mannosidase activity was 171, 124, and 113; and the medianβ-glucuronidase activity was 133.7, 14.3, and 10.0, respectively. The difference of the activities of the four enzymes measured between the bacterial meningitis and the controls is significant (p < 0.000). Also, significant is the difference between bacterial and aseptic meningitis(p = 0.005 to <0.000), but it is not significant between aseptic and control subjects. Both the sensitivity and specificity of theβ-glucuronidase activity between bacterial meningitis and control subjects were 100%, whereas the corresponding values between bacterial and aseptic meningitis were 100% and 90%, respectively. No significant correlation was observed between the activities of the enzymes measured and the number of the polymorphonuclear leukocytes or other laboratory characteristics of the CSF. The increased lysosomal enzyme activities in the CSF of patients with meningitis may result from diffusion across the blood-CSF or the brain-CSF barrier or from enzyme leakage through the cell membranes.
Similar content being viewed by others
Main
The lysosomal enzymes are glycoproteins, and their precursors are synthesized in ribosomes bound to the rough endoplasmic reticulum(1). The targeting of these enzymes from their site of synthesis to their final destination in lysosomes is controlled by a series of protein and carbohydrate recognition signals localized on the enzyme molecule(2–4).
Both BM and AM cause inflammation of the meninges, ventriculitis, vascular and parenchymal cerebral changes, and CSF pleocytosis. These abnormalities are more profound in BM than in AM.
Inflammation could affect the sorting, targeting, packaging, or the salvage pathway of the lysosomal enzymes(2–5). Any such derangement of the lysosomal enzymes' trafficking could lead to enzyme leakage through the cell membranes and the presence of increased concentrations of lysosomal enzymes in the CSF. Also, inflammation could lead to an increased formation of phagosomes that engulf endocytized microorganisms and other foreign materials. Subsequently, these phagosomes by fusing with lysosomes initiate digestion, followed by expulsion of the vacuoles' residual contents, including the remaining lysosomal enzymes, to the extracellular space. It has been shown that lysosomal enzymes may be released from cytoplasmic granules into a free or nonsedimental form during the early course at autolysis(6). In addition, during inflammation the lysosomal enzymes may cross, in an increased rate, the blood CSF and the brain-CSF barriers, thus appearing in greater concentrations in the CSF.
There are several reports indicating that inflammation is associated with PMN leukocyte infiltration and release of lysosomal enzymes. Thus increased concentrations of lysosomal enzymes in extracellular fluids has been found in experimentally induced inflammation(7–11), as well as in patients with various inflammatory disorders, such as in rheumatoid synovial fluids(12), pulpal inflammatory disease(13), and gingival crevicular fluid(14). Also, elevated activities of the lysosomal enzymesβ-hex and α-mann have been measured in the plasma of patients with the human immunodeficiency virus disease(15).
Increased concentrations of lactic dehydrogenase in the CSF have been found in patients with meningitis, especially of bacterial etiology(16–18). Particularly, the isozymes 1, 2, and 3 appear mainly in viral meningitis, whereas the isozymes 4 and 5 predominate in BM. Increased β-gluc activity has been found in CSF containing cellular elements of a patient with partially treated pyogenic meningitis, whereas in another such patient it was normal(19). Similarly, of four viral meningitis cases studied, in two the activity was increased, whereas in the other two it was within the normal range(19).
For determining whether or not the levels of the lysosomal enzymes are increased in the cell-free CSF of patients with meningitis, we measured the activity of four such enzymes in the cell-free CSF of children with BM and AM, as well as of febrile children without CSF pleocytosis.
METHODS
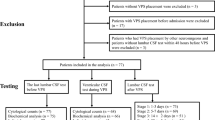
Patients and control subjects. A total of 54 patients with meningitis who were admitted to the pediatric wards of the two participating centers between January 1990 and March 1996 were investigated. Thirty-four of them were acute BM and 20 AM. The median age of the BM cases was 3 9/12 y(range 6 mo to 13 y) and of the AM it was 6 7/12 y (range 1 1/12 to 14 y). In addition, one patient with meningococcal sepsis and borderline CSF pleocytosis(12 cells/mm3 CSF, positive CSF and blood cultures), as well as two cases of meningococcal sepsis, without CSF pleocytosis (3 and 6 leukocytes/mm3, positive blood cultures) were studied.
Table 1 lists the CSF laboratory findings of the meningitis cases studied. All patients with BM included in the study were diagnosed with a positive CSF culture or a positive CSF latex particle agglutination test. Twenty one of the cases were due to Neisseria meningitidis (16 positive CSF culture, five positive CSF latex), nine toHaemophilus influenzae type b (eight positive CSF culture, one positive CSF latex) and four to Streptococcus pneumoniae (three positive CSF culture, one positive CSF latex).
In the cases of AM there was CSF pleocytosis and absence of microorganisms on Gram stain and culture, and no antigen was detected in the CSF by the latex particle agglutination test. Serology was negative for toxoplasmosis, brucellosis, and salmonellosis. All patients became ill abruptly and were febrile, and the clinical symptoms were typical of meningitis or meningoencephalitis. All cases recovered without antibiotic therapy. No malignancy was identified in any of the cases. In two patients (13 and 22 mo old) with CSF pleocytosis, Kawasaki disease was diagnosed. The first patient was followed up with three additional lumbar punctures performed over a period of 14 d. Two cases developed after mumps infections that were verified by serology. In one case with herpes zoster meningoencephalitis, on the 5th day after the appearance of the vesicular rash, the IgM and IgG titers of the anti-varicella antibodies were 63 and 118 EU/mL, respectively. Eight days later the titers were 120 and 143, respectively.
The CSF specimens obtained from 39 febrile patients suspected of having meningitis, but found to have no CSF pleocytosis, were used as controls. The median age of the control subjects was 3 mo, with a range from 3 d to 3 6/12 y. In addition, from 10 of the control subjects, blood serum was obtained for enzyme measurements.
Informed consent was obtained from the parents of the patients and the control subjects. The study was approved by the Ethics Committee of the General University Hospital of Patras.
Samples. The mean ± SD time lapsed from the onset of clinical symptoms of BM to lumbar puncture was 23 ± 11 h, with a range from 7 to 40 h. In AM it was 1.7 ± 1.3 d, range 10 h to 4 d. A portion of the CSF of each case was centrifuged within 20 min from the time of the lumbar puncture, and the supernatant cell-free fraction was stored at-70°C until assayed.
The activity of the enzymes studied was determined by incubating the CSF with the appropriate fluorogenic substrate for 1 h at 37°C. The reactions were stopped with the addition of 0.085 M glycine-carbonate buffer, pH 10.5(stopping buffer). The reactions were carried out in plastic tubes, 100× 8 mm (V.I.V.E. Anapliotis S.A., Nea Philadelphia, Greece).
The β-hex A and B (EC 3.2.1.30) activity was measured according to the method of Okada and O'Brien(20) by using 4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-glucopyranoside (Koch-Light Laboratories Ltd, Colnbrook Bucks, England) as substrate.
α-Mann (EC 3.2.1.24) was determined by using 4-methylumbelliferyl-α-D-mannopyranoside (Koch-Light Laboratories, Ltd) as substrate. The reaction tube contained 25 μL of CSF and 100 mL of buffered substrate (8 mM substrate in 0.1 M citrate-phosphate buffer, pH 4.0). The reaction was stopped with the addition of 1.8 mL of stopping buffer.
β-Gluc (EC 3.2.1.31) was measured by incubating 200 μL of CSF with 50 μL of buffered substrate containing 4 mM 4-methylumbelliferyl-β-D-glucuronide (Sigma Chemical Co., St. Louis, MO) in 0.1 M acetate buffer, pH 4.0. The reaction was stopped with 5 mL of stopping buffer.
All assays were performed in duplicate. The released 4-MU was determined against a blank and standard 4-MU solutions. The fluorescence was measured with a Sequoia-Turner fluorometer (model 450; Sequoia-Turner Corp., Mountain View, CA) at 450 nm after excitation at 360 nm. Activity is expressed in nanomoles of 4-MU/mL of CFS/h.
Statistical analysis. The Wilcoxon signed ranks test was applied for the statistical analysis of the data. The Bonferroni correction was used when multiple comparisons were made. The Spearman correlation was used to test the significance of correlations between enzyme activities and laboratory characteristics of the CSF or the age of the patients. Significance was set at 0.05.
RESULTS
The median β-hex A activity in the CSF of the patients with BM was 313, in AM it was 173 and in the control subjects it was 175(Fig. 1). The difference between BM and control subjects is significant (z = -4.402, p < 0.000). Also, significant is the difference between BM and AM (z = -2.800,p = 0.005). However, the difference between AM and control subjects is not significant (Bonferoni correction: 0.05 divided by 3, statistical significance at p < 0.017).
β-Hex A activity in 34 BM patients, 20 AM patients, and 39 control subjects without CSF pleocytosis. Cases 1 and 2, Kawasaki disease; 3 and 4, mumps; 5, herpes zoster meningoencephalitis. Cases a, b, and c, BM patients with the lowest β-gluc activities. Horizontal bars, 4 SD above the mean activity of the control cases.
In the CSF of the patients with BM the median β-hex B activity was 417, in AM it was 165, and in the control subjects it was 120(Fig. 2). The difference between BM and control subjects, as well as between BM and AM is significant (z = -4.867, p< 0.000; and z = -3.173, p = 0.002, respectively). The difference between AM and controls is not significant (Bonferroni correction, significance at p ≤ 0.017).
β-Hex B activity in 34 BM patients, 20 AM patients, and 39 control subjects without CSF pleocytosis. Symbols as in Figure 1.
The median α-mann activity in the CSF of the patients with BM, AM, and the control subjects was 171, 124, and 113, respectively (Fig. 3). The difference between BM and control subjects is significant (z = -4.163, p < 0.000); it is significant as well between BM and AM (z = -3.846, p < 0.000). The difference between the patients with AM and the control subjects is not significant (Bonferoni correction, significance at p ≤ 0.017).
α-Mann activity in 34 BM patients, 20 AM patients, and 39 control subjects without CSF pleocytosis. Symbols as in Figure 1.
The median β-gluc activity in the CSF of the patients with BM was 133.7 (range 44.0 to 826.0), in the patients with AM it was 14.3 (range 5.0 to 44.8), and in the control subjects it was 10.0 (range 3.8 to 24.8)(Fig. 4). The difference of the enzyme activity between BM and control subjects is significant (z = -5.086, p < 0.000). Similarly, the difference between BM and AM is significant(z = -3.920, p < 0.000), but there is no significant difference between AM and control subjects (Bonferroni correction, significance at p ≤ 0.017).
β-Gluc activity in 34 BM patients, 20 AM patients, and 39 control subjects without CSF pleocytosis. Symbols as in Figure 1.
In three BM cases with 30, 56, and 60 cells/mm3 CSF the β-gluc activity was 117.6, 46.0, and 71.8; the α-mann was 188, 62, and 151; theβ-hex A was 187, 224, and 252; and the β-hex B was 346, 229, and 244, respectively. All three cases were due to N. meningitidis.
In the patient with meningococcal sepsis and the presence of 12 cells/mm3 in the CSF, the β-gluc, the α-mann, and theβ-hex A and B activities were 26.2, 42, 58, and 96, respectively. In the two cases of meningococcal sepsis, without CSF pleocytosis, the enzyme activities were 20.3, 25, 445, and 463, and 30.0, 54, 127, and 132, respectively.
The β-gluc and α-mann activities in the CSF of the initial lumbar puncture and the three subsequent ones performed 2, 7, and 14 d after the initial puncture in one of the two patients with the Kawasaki disease were 70, 60, 60, 36, and 145, 150, 260, 96 for each of the two enzymes, respectively. The cell number in the CSF of the first and the three follow-up punctures was 140 (100% MN), 50 (2% PMN, 98% MN), 68 (9% PMN, 91% MN), and 8, respectively.
The sensitivity and specificity of the activity of the four enzymes measured in the CSF of the BM cases compared with the control CSF without pleocytosis and to the enzyme levels in the CSF of the AM cases are listed in Table 2.
There was no correlation between the β-gluc activity in the CSF and the age of both the children without CSF pleocytosis and the meningitis patients, bacterial or aseptic. Also, there was no significant difference in the β-gluc activity between the children with BM from 6 mo to 3 y of age(median 143.0, range 46.0 to 398.4) and from 3 to 13 y of age (median 130.0, range 43.9 to 826.0). When 16 patients with BM and 10 control subjects of a similar age were compared (median 1.5 y, range 0.5 to 3 y, and median 1.3 y, range 0.7 to 3 y, respectively), the β-gluc activity was again significantly greater in the meningitis cases (median 175.8, range 46.0 to 398.4) than in the children without CSF pleocytosis (median 9.3, range 6.9 to 24.8) (z = -2.803, p < 0.000). Similar findings were observed with the other three enzymes that were studied.
The activities of the four lysosomal enzymes measured were correlated with the laboratory characteristics of the CSF from the patients with BM and AM listed in Table 1. No significant correlation was observed between the enzyme activities and the number of the PMN cells in the CSF (Table 3) or with any other laboratory characteristic of the CSF.
Table 4 lists the mean activity of the four lysosomal enzymes measured in both the blood serum and the CSF of 10 children without CSF pleocytosis as well as the ratios between the enzyme activities in the serum and the CSF.
DISCUSSION
The findings of the study demonstrated increased activities of the lysosomal enzymes β-hex A and B, α-mann, and β-gluc in the cell-free CSF of patients with BM when compared with the activities of these enzymes in children with AM or with febrile illnesses, but without CSF pleocytosis.
Of the four lysosomal enzymes investigated, β-gluc activity provided the best separation between BM on the one hand, and AM or control cases without CSF pleocytosis on the other. In BM the activity was always greater than in the control subjects, whereas in one patient with BM it was slightly lower and in two other patients only slightly above the upper range of the AM cases. The elevation of the β-gluc activity in CSF pleocytosis of bacterial etiology was observed even when the number of cells in the CSF was lower than 100/mm3. It should be noted, however, that in one out of three such cases the enzyme activity was close to that measured in the CSF of two patients with AM, one with herpes zoster and an other one of unknown etiology. It is worth mentioning that, in the patient with borderline CSF pleocytosis, 12 cells/mm3, and the case of meningococcal sepsis with 6 cells/mm3, the β-gluc activity, although within 4 SD above the normal mean, was greater than the upper range of the activities measured in the control CSF without pleocytosis. However, in the patient with meningococcal sepsis and 4 cells/mm3 in the CSF the β-gluc activity was within the normal range. These findings indicate that theβ-gluc activity in the CSF is a sensitive index of BM, even when the cell number in the CSF is below 100/mm3, and the other laboratory parameters are unaffected.
The BM case in which the β-gluc activity overlapped with the upper range of the AM cases was due to S. pneumoniae; the CSF leukocyte count was 11 800/mm3, with 95% of the cells PMN. Although the activities of all acid hydrolases measured in this case were low, it should not be considered as a characteristic of pneumonococcal meningitis because in three other such cases studied the β-gluc activity was 500, 326, and 173.
Because of the broad overlapping of the α-mann activity in the CSF of the patients with BM, AM, and the control subjects without CSF pleocytosis, only the high activities of this enzyme can be interpreted as a positive finding, indicating meningeal inflammation of bacterial etiology.
The distribution of the β-hex A and B activities showed a similar pattern in the groups of subjects investigated. It is worth mentioning that the activities of these enzymes in the CSF of some patients with AM were well above the median activity found in the patients with BM.
From the data collected so far it appears that the parallel measurement of the activity of the four enzymes studied will not increase the diagnostic power of the method because in the three cases of BM, with the lowestβ-gluc activities, the levels of the three other enzymes measured were within the normal range.
Although in some of the AM cases the activity of the lysosomal enzymes studied in the CSF was greater than in the control subjects without CSF pleocytosis, the validity of these enzyme measurements for the distinction between the two groups of subjects is limited. The best separation between AM and control subjects was obtained with the measurement of the β-hex B activity. In one-fourth of the AM cases studied the activity of this enzyme was greater than 4 SD above the mean enzyme level in the control subjects. A parallel measurement, therefore, of the β-hex B and the β-gluc activity could identify approximately one-fourth of the AM cases from the normal subjects and the patients with BM, without taking into account any other parameters from the CSF. Such patients would show increased β-hex B activity, whereas the β-gluc activity would remain within the normal range. Alternatively, cases with CSF pleocytosis and β-gluc activity within the normal range should be considered, with a high degree of accuracy, of nonbacterial origin.
With the exception of the two cases of mumps, the case of herpes zoster and the two cases of Kawasaki disease, the exact nature of the remaining 14 AM cases is uncertain. However, a viral etiology is very likely because of the clinical picture, that included a high fever, and the fact that all patients recovered without antibiotic treatment.
Although the control subjects were unmatched for age with the meningitis cases, the observed differences in the enzyme activities between patients and control subjects without CSF pleocytosis cannot be attributed to this age difference because: 1) no correlation was found between enzyme activities in the CSF of the control subjects and age, 2) there was no significant difference in the enzyme activity between the BM patients with an age of 6 mo to 3 y and 3 to 13 y, and 3) the enzyme activity in patients with BM was significantly greater than in age-matched control subjects.
The increased activities of the lysosomal enzymes measured in the cell-free CSF of the patients with CSF pleocytosis could originate from enzyme diffusion across the blood-CSF and the brain-CSF barriers, or from cells present in the CSF. Because no significant correlation was found between cell number and enzyme activity in the CSF, it is unlikely that the increased activities measured in the patients with CSF pleocytosis resulted from the cellular elements within the CSF. Most probably the increased enzyme concentrations originated from brain or meningeal tissue and from diffusion across the blood-CSF barrier. Inflammation could cause a disturbance of the trafficking of the lysosomal enzymes, autolysis, or cellular death, all of which would lead to enzyme leakage through the cell membranes. Inflammation has been shown to cause increased concentrations of lysosomal enzymes in extracellular fluids(7–14). A differential response of two lysosomal enzymes during the early phases of experimental granulomatous inflammation has been reported(21). The observation that the blood to CSF ratio was greatest for β-gluc may also explain the better distinction between BM and control subjects or AM afforded by this enzyme; bacterial infections cause a more severe inflammation and, therefore, a greater permeability of the meningeal membranes.
Measurement of the β-gluc activity in the CSF has been carried out in a number of neurologic disorders(18), as well as in two partially treated cases of purulent meningitis, in viral meningitis, and in a number of nonviral AM cases, such as cryptococcal, tuberculous, toxoplasmic, and syphilitic cases(22). With the exception of the cryptococcal meningitis, there was a general increase in the enzyme activity, with a broad overlapping between the groups of patients studied and the control subjects. However, in these studies CSF containing the cellular elements was used, and therefore, the enzyme activity measured in each case was greatly influenced by the number and the type of cells present in the CSF. In addition, for the enzyme measurement a chromogenic substrate requiring an 18-h incubation period was applied. Nonetheless, in samples with low activity the absorbance of the final product was below optimal range, and the activity was evaluated against a nonlinear standard curve. The fluorogenic substrate used in this study required an incubation period of only 1 h, the fluorescence released was well above the border of the fluorometer's sensitivity, and the 4-MU standard curve was linear.
A bacterial origin of the increased enzyme concentrations found in the CSF of patients with meningitis cannot be supported from existing data.β-Gluc is rarely produced by H. influenzae(23). Activity of this enzyme has been found in types ofStreptococcus pyogenes(24),Streptococcus mitis(25), andStreptococcus agalactiae(26), as well as in strains of Escherichia coli and Shigella species(27). Against a bacterial origin of the increased enzyme activities found in the CSF of BM are the observations that there was a general increase in the activity of all enzymes studied, as well as that there was no significant difference in the enzyme activity among the patients with meningitis due to the three different etiologic agents identified in the CSF.
In conclusion, the activity of four lysosomal enzymes is elevated in the cell-free CSF of patients with BM meningitis. The β-gluc activity shows the greatest increase in BM and, therefore, measurement of this enzyme in the CSF provides an easy and fast method for the early diagnosis of this severe and frequently life-threatening disease. The high sensitivity and specificity of the procedure indicates that the β-gluc activity in the CSF will become a useful tool in the evaluation of patients with meningitis and in reaching an early decision about the proper course of treatment to be followed.
Abbreviations
- AM:
-
aseptic meningitis
- BM:
-
bacterial meningitis
- CSF:
-
cerebrospinal fluid
- β-gluc:
-
β-glucuronidase
- β-hex:
-
β-hexosaminidase
- α-mann:
-
α-mannosidase
- MU:
-
methylumbelliferone
- MN:
-
mononuclear
- PMN:
-
polymorphonuclear
References
Kornfeld S 1986 Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest 77: 1–6
Erickson AH, Blobel G 1979 Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem 254: 11771–11774
Rosenfeld MG, Kreibich G, Popov D, Kato K, Sabatini DD 1982 Biosynthesis of lysosomal hydrolyses: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol 93: 135–143
Proia RL, Neufeld EF 1982 Synthesis ofβ-hexosaminidase in cell-free translation and in intact fibroblasts: an insoluble precursor α chain in a rare form of Tay-Sachs disease. Proc Natl Acad Sci USA 79: 6360–6364
Vladutiu GD, Rattazzi M 1979 Excretion-reuptake route ofβ-hexosaminidase in normal and I-cell disease cultured fibroblasts. J Clin Invest 63: 595–601
Van Lancker JL, Holtzer RL 1959 Release of acid phosphatase and β-glucuronidase from cytoplasmic granules in early course of autolysis. Am J Pathol 35: 563–573
Brielans JK, Kunkel RG, Fantone JC 1987 Pulmonary alveolar macrophage function during acute inflammatory lung injury. Am Rev Respir Dis 135: 1300–1306
Kaga S, Kobayashi K, Yamagata N, Takeuchi HT, Yoshida K, Matsuda T, Nakatani K, Kasama T, Kasahara K, Takahashi T 1991 Lysosomal enzyme activities in experimental granulomatous inflammation. Int Arch Allergy Appl Immunol 95: 236–243
Safina A, Korolenko T, Mynkina G, Dushkin M, Krasnoselskaya G 1992 Immunomodulators, inflammation and lysosomal proteinases of macrophages. Agents Actions Suppl 38: 191–197
Safina AF, Korolenko TA, Mynkina GI, Dushkin MI, Krasnoselskaya GA 1992 Liver and serum lysosomal enzymes activity during zymosan-induced inflammation in mice. Agents Actions Suppl 38: 370–375
Hubard AK, Vetrano KM, Morris JB, Thrall RS 1994 Acute NO2 exposure alters inflammatory cell activation and particle clearance in silica-injected mice. J Toxicol Environ Health 41: 299–314
Shingu M, Todoroki T, Nobunaga M 1987 Rheumatoid synovial fluids generated significantly greater amounts of superoxide, lysosomal enzymes, and superoxide dismutase from neutrophils into extracellular fluid than osteoarthritic synovial fluids. Inflammation 11: 143–151
McClanahan SB, Turner DW, Kaminski EJ, Osetek EM, Heuer MA 1991 Natural modifiers of the inflammatory process in the human dental pulp. J Endod 17: 589–593
Lamster IB, Novak MJ 1992 Host mediators in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. Crit Rev Oral Biol Med 3: 31–60
Lügering N, Stoll R, Siekman A, Faulhaber J, Heese C, Dietrich O, Kucharzik T, Busch H, Hasilik A, Domschke W 1995 Elevated levels of activities of β-hexosaminidase and α-mannosidase in human immunodeficiency virus-infected patients. J Infect Dis 171: 683–686
Feldman WE 1975 Cerebrospinal fluid lactic acid dehydrogenase activity: levels in untreated and partially antibiotic-treated meningitis. Am J Dis Child 129: 77–80
Neches W, Platt M 1968 Cerebrospinal fluid LDH in 287 children, including 53 cases of meningitis of bacterial and non-bacterial etiology. Pediatrics 41: 1097–1103
Nelson PV, Carey WF, Pollard AC 1975 Diagnostic significance and source of lactate dehydrogenase and its isoenzymes in cerebrospinal fluid of children with a variety of neurological disorders. J Clin Pathol 28: 828–833
Shuttleworth EC, Allen N 1968 Early differentiation of chronic meningitis by enzyme assay. Neurology 18: 534–542
Okada S, O'Brien JS 1969 Tay-Sachs disease: generalized absence of β-D-N-acetylhexosaminidase. Science 165: 698–700
Lesser M, Chang JC, Galicki NI, Edelman J, Cardozo C 1989 Cathepsin B and D activity in alveolar macrophages from rats with pulmonary granulomatous inflammation or acute lung injury. Agents Actions 28: 264–271
Allen N, Reagan E 1964 β-Glucuronidase activities in cerebrospinal fluid. Arch Neurol 11: 144–154
Kilian M 1976 A taxonomic study of the genusHaemophilus, with the proposal of a new species. J Gen Microbiol 93: 9–62
Williams REO 1954 Glucuronidase production by serotypes of Streptococcus pyogenes. J Gen Microbiol 10: 337–344
Nord C-E, Linder L, Wadström T, Lindberg AA 1973 Formation of glycoside-hydrolases by oral streptococci. Arch Oral Biol 18: 391–402
Röd TO, Haug RH, Midtvedt T β-Glucuronidase in the streptococcal groups B and D. Acta Pathol Microbiol Scand Sect B 82: 533–536
Kilian M, Bülow P 1976 Rapid diagnosis of Enterobacteriaceae. I. Detection of bacterial glycosidases. Acta Pathol Microbiol Scand Sect B 84: 245–251
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Beratis, N., Mavrommatis, T., Hatiris, I. et al. Increased Activity of Lysosomal Acid Hydrolases in the Cell-Free Cerebrospinal Fluid of Bacterial Meningitis. Pediatr Res 41, 235–241 (1997). https://doi.org/10.1203/00006450-199702000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199702000-00014
This article is cited by
-
Study of the pharmacokinetic and pharmacogenetic contribution to the toxicity of high-dose methotrexate in children with acute lymphoblastic leukemia
Medical Oncology (2012)
-
Increased activity of lysosomal enzymes in the peritoneal fluid of patients with gynecologic cancers and pelvic inflammatory disease
Journal of Cancer Research and Clinical Oncology (2005)