Abstract
Group B streptococci (GBS) are one of the major causes of invasive neonatal infection. The pathogenesis of early onset disease is a multistep process. Adhesion of GBS to eucaryotic cells is considered to be an important step for the establishment of infection. Subsequent to adhesion, GBS invade cells and give rise to septicemia and meningitis. To investigate passage of GBS across epithelial cell linings we examined the interaction between bacteria and Madin-Darby canine kidney (MDCK) cells. When grown on permeable support, these cells form a polarized epithelial monolayer with an apical-to-basolateral orientation, which more reflects the in vivo situation compared with conventionally cultured cells. Our results show that GBS are translocated in vacuoles from the apical to the basolateral surface of MDCK cells in a temperature-dependent process. The passage of GBS through the cells is selective with only small numbers of bacteria penetrating in the basolateral-to-apical direction. Transcytosis of GBS starts before decrease in transepithelial resistance of the monolayer. These data suggest a mechanism for traversal of GBS over intact chorioamniotic membranes and from alveoli into the circulation of the fetus.
Similar content being viewed by others
Main
GBS are one of the most common causes of invasive neonatal infection with an incidence of 0.3-4 cases per 1000 live births(1–4). Any serotype of GBS can be involved, but serotype III causes one-third of early onset cases and the majority of late onset cases(5). The pathogenesis of early onset disease is a multistep process with vertical transmission from mother to offspring(6). Mothers colonized with GBS in the urogenital tract can spread the bacteria to the fetus during labor or even across intact chorioamniotic membranes(7, 8). The fetus aspirates the infected amniotic fluid. The lungs are the the main primary target, and pneumonia is observed in the majority of early onset infections(9, 10). GBS subsequently spread across the respiratory epithelium and vascular endothelium to give rise to septicemia and meningitis(6). However, details of this pathogenic scenario are not completely defined. GBS can invade the pulmonary epithelium, interstitial fibroblasts, and lung capillary endothelium in an in vivo newborn primate model of early onset disease(11). GBS adhere to and invade a number of human cellsin vitro, including vaginal, respiratory, and endothelial(12–17). These abilities are considered to be crucial steps for establishment of infection in the neonate.
Considering this pathogenic model, GBS have to cross a number of barriers in the genital and respiratory tracts to reach the blood stream. To investigate passage of GBS across epithelial cells we examined the interaction between bacteria and MDCK cells derived from the kidney of a cocker spaniel. When grown on a permeable support, these cells form a polarized epithelial monolayer with an apical-to-basolateral orientation, which more reflects thein vivo situation compared with conventionally cultured cells(18). Our results show that GBS are translocated within vacuoles from the apical to the basolateral surface of MDCK cells.
METHODS
Bacterial strains. Ten group B streptococcal strains (I-X) isolated at the Department of Clinical Microbiology, Medical Center Hospital,Örebro, Sweden (Table 1), and 8 GBS reference strains (U1-U8)(15) were included in the study. Of the latter 8 strains, 6 were serotype III (variants U1-U6). These were selected in pairs as isogenic variants with high and low ability to form capsules. The amount of capsular substance has been determined by measuring sialic acid by the thiobarbituric assay and by density profiles by buoyant density(19, 20). Variants U1, U3, and U5 form high amounts of capsule, and they were originally from the same clinical isolates as the variants forming low amounts of capsule, U2, U4, and U6, respectively. Strains U7 and U8 were nontypable and formed no capsule. Salmonella typhimurium 395 MR10, a rough lipopolysaccharide mutant(21) and Escherichia coli DH5α (Bethesda Research Laboratories, Gaithersburg, MD) were used as internal standards in bacterial penetration of cells. All strains were kept at -70°C and grown on GC agar (GC agar medium base, BBL. Cockeysville, MD, supplemented with 1% Hb. 10% horse serum, and 1% Isovitalex) at 36°C in 5% CO2 for 18-24 h.
Cell culture. MDCK strain 1 cells were used between passage 5 and 80. Cells were grown in minimal essential Eagle's medium with Earle's salt(ICN Biomedical Inc., Costa Mesa, CA) supplemented with 10% FCS, 2 mM glutamine, 1% nonessential amino acids, 10 mg/L gentamicin) GM. Cells were passed twice weekly. Millicell filter units (Millipore Corp., Bedford, MA) with a 0.6-cm2 porous filter membrane with 0.4 or 3.0-μm pores, were coated with collagen type I (Sigma Chemical Co., St. Louis, MO). Collagen was diluted in ethanol to 0.75 g/L, and 50 μL of the solution were added to filters and allowed to dry overnight. Filter units were placed in 24-well plates (Nunclone, Nunc, Roskilde, Denmark) containing GM to completely moisten the filters. Preincubation medium was removed, and 0.5 mL of GM with 5 × 105 MDCK cells/mL were added. The plates were incubated in 5% CO2 at 37°C for 3-4 d until the monolayer reached confluency. Medium was changed each day, the last changed without gentamicin.
Association assay. Association of bacteria with MDCK cells was performed with cells grown on 0.4-μm filters. Bacteria were suspended in GM without gentamicin to approximately 1 × 108 bacteria/mL as determined by the McFarland scale, calibrated repeatedly by counting in a Bürker chamber. The concentration of bacteria was verified for each experiment by viable count. All GM was carefully removed from the cells, and 300 μL of the bacterial suspension were added to the apical surface of the cells. The plates were incubated in 5% CO2 at 37°C for 2 and 4 h. Then the cells were rinsed five times with antibiotic-free GM, the filter units were transferred to empty wells, 0.5 mL of 10% Triton-X 100 in PBS was added, and incubation was for 30 min at 37°C. The lysate was diluted in antibiotic-free GM, and 10 μL of appropriate dilutions were incubated on GC agar at 36°C overnight. The number of colony-forming units was counted, and the association of bacteria was expressed as percent of inoculum. The Triton X-100 solution did not affect bacterial viability. Corresponding experiments were also performed with collagen-coated filters without MDCK cells to determine background adherence.
Penetration assay. MDCK cells were cultured on collagen-coated 3.0-μm filters for 3-4 d until confluency. The medium was removed, and 400μL of 1 × 108 bacteria/mL in GM without gentamicin were added to the apical cell surface and 600 μL of medium to the outer well. Filter units were incubated in 5% CO2 at 37°C for the indicated times. At each time point, 10 μL of medium were removed from the outer well, and the number of bacteria was titrated by plating on GC agar. Unless otherwise stated, the filter units were transferred after 1, 2, 3, 4, 6, 8, 10, and 12 h of incubation to fresh 24-well plates containing 600 μL of antibiotic-free GM per well. As control experiments, bacteria were added to the outer well to study penetration from the basolateral cell surface and to filters without MDCK cells. Cell viability was determined by exclusion of 0.2% trypan blue.
Measurements of electrical resistance. The transepithelial electrical resistance of MDCK cell monolayers was measured by a Millicell-ERS apparatus according to instructions of the supplier (Millipore). The resistance was expressed as Ωcm2 by multiplying the measured resistance by the area of the filter.
Transmission electron microscopy. MDCK cells grown on 3.0-μm pore size filters and infected with GBS from the apical side were washed several times with PBS and fixed in 2% glutaraldehyde in 0.1 M sucrose/sodium cacodylate-HCl buffer, pH 7.2, at 4°C overnight. Samples were postfixed in 1% OsO4 in 0.15 M sodium cacodylate-HCl buffer for 90 min, dehydrated in a series of ethanol, and stained with 2% uranyl acetate in 50% ethanol overnight. Cells were embedded in Epon 812 (Fluka AG, Buchs, Switzerland), sectioned, and stained with lead citrate before examination at 100 kV in a JEOL 2000-Ex (Tokyo, Japan) electron microscope.
RESULTS
Association of GBS with MDCK cells. GBS were added to the apical surface of MDCK cells, and the number of bacteria associated with the cells was determined after 4-h incubation by viable count of host cell lysates. Of the clinical isolates, 0.7-4.3% of serotype III strains,i.e. I, VII, and IX associated with the cells(Fig. 1). The other serotypes, except number X, a serotype Ia strain, interacted to a much lesser extent. After 2-h incubation a significantly lower association was observed; 0.016, 0.11, and 0.13% of GBS V, VI, and VII, respectively, associated with the cells compared with 0.18, 0.53, and 2.25% after 4-h incubation. Thus, the differences between strains with high and low association after 4 h were readily observed after 2 h of incubation. To investigate a possible correlation between expression of capsule substance and association with MDCK cells, isogenic variants of reference strains (U1-U6) were investigated. Strains U1 and U5, both with a high amount of capsule substance, associated with MDCK cells to a lower extent compared with their isogenic variants U2 and U6 (Fig. 1). On the other hand, strain U3 showed a higher association compared with U4. Thus, there was no obvious difference in association with the cells between variants with high and low expression of capsule. This is in contrast to interaction with, e.g. endothelial cells where capsule expression impairs binding of GBS to the cells(15). The mean generation times for the strains grown in GM without gentamicin were 31-48 min during 4 h of incubation. No correlation between generation time and magnitude of association with MDCK cells was observed.
The adhesion of clinical isolates and reference strains to collagen-coated filters without MDCK cells varied from 0.001 to 0.3% and was for most strains only a fraction of the association with MDCK cells. Strains that showed high association with MDCK cells often showed higher adherence to the collagen-coated filters compared with those that had low association values to MDCK cells. Thus, a correlation between adherence to MDCK cells and collagen-coated filters was observed.
Penetration of GBS through polarized MDCK cell monolayers. By growing MDCK cells on filters with 3.0-μm pores, quantitation of bacterial passage through this barrier is possible. Initial experiments showed that 5-50% of the inoculum of selected GBS isolates passed through filters without MDCK cells at 2 h. As expected, no GBS bacteria passed 0.4-μm filters. A potential drawback with this method is that the polarized cells may form an imperfect monolayer, allowing passage of bacteria through gaps between cells. To control for this, two types of experiments were performed. The transepithelial resistance in confluent uninfected MDCK cell monolayers was 624 ± 136 Ωcm2 (Fig. 2). A similar value has been reported by Finlay et al.(22) and indicates the presence of a highly impermeable monolayer. Furthermore,Escherichia coli DH5α was used as an internal standard for penetration. This strain is noninvasive and is not capable of passing intact MDCK cell monolayers(22). In all experiments less than 500 of added E. coli DH5α passed the monolayer in 12 h(Fig. 3). These experiments show that the MDCK cell monolayers form sealed epithelial barriers.
Total bacterial penetration through MDCK cell monolayers: 4 × 107 colony-forming units (cfu) of GBS clinical isolate IX (□), reference strains U5 (▪), and U6 (▪), S. typhimurium (▪), of E. coli DH5α (▪) were added to the apical cell surface and the number of bacteria in the basolateral medium was determined after 1-24 h. Bars represent mean ± SD of 2-36 experiments.
When added to the apical surface of the monolayers, the number of GBS found in the basolateral medium increased with incubation time. Small numbers of bacteria had penetrated the monolayer after only 1-2 h of incubation(Fig. 3). The number of GBS IX, U5, and U6 penetrating per hour peaked at 10-12 h (Fig. 4). GBS strain I behaved very similarly to strain IX. Interestingly, the encapsulated variant U5 penetrated to a higher extent and with a higher rate compared with the unencapsulated variant U6. This indicates that the capsule may promote bacterial penetration.
Rate of bacterial penetration through MDCK monolayers. Symbols as in Figure 3.
The transepithelial resistance in GBS U5-infected monolayers remained constant at 400-500 Ωcm2 for the first 10 h and did not differ significantly from uninfected cells (Fig. 2). After 24 h the resistance decreased to 225 ± 71 Ωcm2. Thus, GBS penetrated the monolayers before a significant drop in trans-epithelial resistance occurred. Despite this drop in resistance, infected cells remained viable. Trypan blue was excluded from >95% of GBS-infected cells after 4 h. Twenty-four hours after inoculation of the apical surface, 10% of GBS-infected cells were stained, the same figure as for uninfected cells. In contrast to GBS, Salmonella typhimurium had a slower kinetics of penetration; the bacteria did not pass the monolayers until after 4 h of incubation(Figs. 3 and4). However, after 24 h, a similar number of S. typhimurium and GBS had passed the monolayers. As expected, only a small number of E. coli DH5α penetrated within 12 h. The transepithelial resistance was initially lower in S. typhimurium-infected monolayers compared with those E. coli DH5α-infected, but neither dropped to the values observed in GBS-infected monolayers (Fig. 2).
The effect of temperature on GBS penetration was evaluated. Very few GBS U7 passed the monolayer at 4°C (Table 2). After 24 h of incubation at 22°C, a substantial number of bacteria were found in the basolateral medium, but they were still only about 1% of those penetrated at 37°C. Similar results were found for strain U4 (data not shown).
To determine whether bacterial penetration was a selective apical-to-basolateral passage or if it could occur also in the opposite direction, bacteria were added to the basolateral cell surface. Few GBS U7 andS. typhimurium passed the monolayer during the first 6 h. After 24 h still few GBS U7 were found in the apical medium, whereas a significant number of S. typhimurium had penetrated, albeit less than in the apical-to-basolateral direction (Table 3, Fig. 3). In comparison, 2.1 ± 0.88 × 108 GBS U7 penetrated in the apical-to-basolateral direction at 24 h. GBS strains U1, U2, U3, and U4 behaved similarly to U7 in this respect (data not shown). Because the MDCK cell monolayers were oriented with the apical surface upward also in these experiments, the possibility that gravity significantly affected the failure of GBS to penetrate in the basolateral-to-apical direction was considered. However, GBS U7 and S. typhimurium vigorously penetrated in that direction through filters without MDCK cells (Table 3).
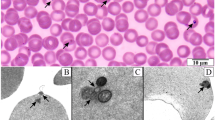
Transmission electron microscopy was used to study the route of GBS penetration. Tight junctions were preserved 6-24 h after inoculation(Fig. 5A) but intercellular spaces were sometimes widened. Intracellular bacteria were enclosed in membrane-bound vacuoles(Fig. 5B). After 6 h of incubation, vacuoles often contained 2-4 bacteria. MDCK cells projected into the pores of the filters, and extracellular GBS were located in these pores at the basolateral surface(Fig. 5C). Occasionally bacteria seemed to reside between cells (not shown).
Transmission electron micrographs of MDCK cell monolayers infected from the apical surface with GBS U7 and incubated for 6 h.(A) Two adjacent cells with intact tight junction (arrow).(B) Two bacteria in a membrane-bound vacuole close to the basolateral surface (arrows). (C) MDCK cells projecting into the 3.0-μm pores of the filters with extracellular bacteria within the pore (arrows). Bars: 0.5 μm (A and B. 1.0μm (C).
DISCUSSION AND CONCLUSIONS
The adhesion to eucaryotic cells is an important step in the pathogenesis of GBS infections. Bacteria adhere to cells that are targets in the course of natural infection, including vaginal(12), pulmonary epithelial(17), and endothelial cells(15). Subsequent to adhesion, GBS invade these cells to occupy an intracellular niche(13, 14, 23). To reach the blood stream and meninges GBS have to cross a number of barriers in the genital and respiratory tracts. Ultrastructural studies by Galask et al.(8) have suggested that GBS may cross the chorioamniotic membrane from the maternal to the fetal surface. However, they provided no data on the magnitude or mechanism of penetration. We have addressed these questions and investigated adhesion to and penetration of GBS through polarized MDCK cells.
GBS serotype III strains associate with MDCK cells to a higher extent than other serotypes, whereas the amount of serotype III capsular polysaccharide does not affect association. Tamura et al.(17) have recently found low levels of adherence of a serotype III strain to MDCK cells compared with adhesion to human epithelial cells. However, they grew cells on plastic support which does not promote an apical-to-basolateral polarization. Considering the different properties of the apical surface compared with the basolateral it is expected that cellular polarization will interfere with bacterial adhesion(24). The higher association with the MDCK cells compared with adhesion to collagen-coated filters suggests a selective binding to the apical cell surface. Bacterial surface molecules that mediate adhesion of GBS to epithelial cells are not completely defined. In contrast to association with MDCK cells, the amount of serotype III capsular substance attenuates binding to several other cells(12, 15, 17). The capsule may block exposure of surface components, such as lipoteichoic acid and proteins implicated in adhesion(25, 26). Bacteria exposed to an acid pH, physiologic in the vagina, show increased adherence(17) and produce low amounts of capsule(27). This indicates that GBS produce low levels of capsular substance at the portal of entry in the genital tract that will promote adhesion. After spreading to the circulation, capsular formation can increase at the close to neutral pH, and phagocytosis of unopsonized GBS diminishes(28).
GBS penetrate MDCK cell monolayers that form a sealed barrier. The transepithelial resistance remains unchanged during the early phases of bacterial penetration, but eventually drops significantly. Such changes in transepithelial resistance is not observed in monolayers infected withS. typhimurium and E. coli DH5α. S. typhimurium significantly penetrates MDCK cell monolayers, but at a slower rate compared with GBS. Notably, E. coli DH5α penetrates very slowly and to a low extent. This indicates that loss of resistance does notper se allow bacterial penetration. Rather, properties of GBS separate from those that cause a drop of resistance appear to determine their ability to penetrate. Our results on transepithelial resistance inSalmonella-infected monolayers differ from those of Finaly et al.(22), who reported a decrease in resistance. We used a lipopolysaccharide mutant of S. typhimurium with low virulence, but with high ability to adhere and invade eucaryotic cells(21). Finlay et al., on the other hand, used a highly virulent strain of Salmonella choleraesuis. These strain differences may explain the various outcome in resistance values. The passage of GBS does not induce cytotoxic effects as assessed by trypan blue exclusion and transmission electron microscopy, and the cells remain viable for at least 24 h. From our data we conclude that GBS adhere to the cells and are internalized into membrane-bound vacuoles. The vacuoles are translocated to the basal surface of the cells where the bacteria are released.
Our results were obtained with kidney epithelial cells from a dog that may differ from the human tissue that GBS normally would penetrate. However, there are obvious advantages for the use of this cell line because it has been extensively characterized [as reviewed in Eaton and Simons(18) and Simoons and Fuller(24)] and previously successfully used to study penetration of Salmonella bacteria(22). Similar experiments with human polarized cells will elucidate a more universal application of our model system.
The penetration of GBS is temperature-dependent, indicating a metabolically active mechanism. Although a substantial number of bacteria cross the cells after 24 h at 22°C, it is only about 1% of those recovered at 37°C. On the other hand, GBS multiplied to only four times the number of bacteria after 24 h at 37°C compared with 22°C. Thus different multiplication rates at 37°C and 22°C may contribute to, but cannot completely explain, this difference in penetration. Only small numbers of GBS penetrated in the basolateral-to-apical direction. This is in contrast to Salmonella with a substantial basolateral-to-apical penetration, although less than in the opposite direction, a finding also reported by Finlay et al.(22).
Three mechanisms for bacterial traversal of the blood-brain barrier has been suggested, and these may apply also generally to passage of bacteria over epithelial linings(29): 1) transcellular transport within host cell vacuoles, 2) paracellular transport after disruption of tight junctions, and 3) transport within phagocytic cells. The results in this report support GBS penetration by the first mechanism, a transcytosis over epithelial linings that is metabolically dependent and selective with respect to direction. Only occasionally bacteria were observed between cells. We postulate that this represents an apical-to-lateral penetration that occurs without change in transepithelial resistance. Thus, this mechanism of penetration may be a variant of apical-to-basal passage and is included in the term transcytosis.
During transit from the genital tract of the mother to the circulation of the offspring, GBS have to pass several epithelial barriers. Some of these passages will be in the apical-to-basolateral direction, e.g. over the genital mucosa and respiratory epithelium in the alveoli. However, others are in the opposite direction, i.e. basolateral-to-apical,e.g. over the chorioamniotic membrane and pulmonary endothelial cells. Although significantly fewer bacteria penetrate in that direction, ourin vitro model may favor apical-to-basolateral penetration. This is because only a fraction of the basolateral cell surface are accessible to bacteria, and they have to overcome gravity in their passage from the lower to the upper tissue culture well. GBS are at least partly able to overcome this, because a substantial number of bacteria pass through filters without cells.
In conclusion, this report shows that GBS can penetrate intact epithelial linings by transcytosis. The results are in accordance with those obtained using live animals. The model will allow identification of bacterial and host cellular factors that regulate this penetration.
Abbreviations
- GBS:
-
group B streptococci
- MDCK:
-
Madin-Darby canine kidney cells
- GM:
-
growth medium
References
Alistair G, Philip MD 1994; The changing face of neonatal infection: experience at a regional medical center. Pediatr Infect Dis J 13: 1098–1102.
Bennnet R, Eriksson M, Melen B 1985; Changes in the incidence and spectrum of neonatal septicemia during a fifteen year period. Acta Paediatr Scand 74: 687–690.
Tessin B, Trollfors K. Thiringer K 1990; Incidence and etiology of neonatal septicemia and meningitis in western Sweden 1975:1986. Acta Paediatr Scand 79: 1023–1030.
Vesikari T, Isolauri E, Tuppurainen H 1989; Neonatal septicemia in Finland 1981-85. Acta Paediatr Scand 78: 44–50.
Dillon HC, Khare S, Gray BM 1987; Group B streptococcal disease: a 6-year prospective study. J Pediatr 110: 31–35.
Tamura GS, Rubens CE 1994; Host-bacterial interactions in the pathogenesis of group B streptococcal infections. Curr Opin Infect Dis 7: 317–322.
Eickhoff T C, Klein JO, Mortiueet EA 1973; The issue of prophylaxis of neonatal group B streptococcal infection. J Pediatr 83: 1097–1098.
Galask R P. Varner MW, Petzold CR, Wilbur SL 1984; Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol 148: 915–925.
Hemming VG, McCloskey DW, Hill HR 1976; Pneumonia in the neonate associated with group B streptococcal septicemia. Am J Dis Child 130: 1231–1233.
Katzenstein A, Davis C, Braude A 1976; Pulmonary changes in neonatal sepsis due to group B β-hemolytic streptococcus: relation to hyaline membrane disease. J Infect Dis 133: 430–435.
Rubens CE, Raff HV, Jackson JC, Chi EY, Bielitzki JT, and Hillier SL 1991; Pathophysiology and histopathology of group B streptococcal sepsis in Macaca nemestrina primates induced after intraamniotic inoculation: evidence for bacterial cellular invasion. J Infect Dis 164: 320–30.
Granlund-Edstedt M. Sellin M, Holm A, Håkansson S 1993; Adherence and surface properties of buoyant density subpopulations of group B streptococci, type III. APMIS 101: 141–148.
Gibson R L, Lee MK. Soderland C, Chi EY, Rubens CE 1993; Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect Immun 61: 478–485.
Hulse M L, Smith S. Chi EY, Pham A, Rubens CE 1993; Effect of type III group B streptococcal capsular polysaccharide on invasion of respiratory epithelial cells. Infect Immun 61: 4835–4841.
Kallman J, Schollin J. Hakansson S, Andersson A, Kihlstrom E 1993; Adherence of group B streptococci to human endothelial cells in vitro. APMIS 101: 403–408.
Rubens CE. Smith S. Hulse M, Chi EY, van Belle G 1993; Respiratory epithelial cell invasion by group B streptococci. Infect Immun 60: 5157–5163.
Tamura GS. Kuypers JM, Smith S, Raff M, Rubens CE 1994; Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun 62: 2450–2458.
Eaton S. Simons K 1995; Apical, basal and lateral cues for epithelial polarization. Cell 82: 5–8.
Håkansson S, Bergholm AM, Holm S, Wagner B, Wagner M 1988; Properties of high and low density subpopulations of group B streptococci: enhanced virulence of the low density variant. Microbiol Pathogen 5: 345–355.
Håkansson S, Granlund-Edstedt M, Sellin M. Holm S 1990; Demonstration and characterization of buoyant-density subpopulations of group B streptococcus type III. J Infect Dis 161: 741–746.
Kihlström E, Edebo L 1976; Association of viable and inactivated Salmonella typhimurium 395 MS and MR 10 with HeLa cells. Infect Immun 14: 851–857.
Finlay B B, Gumbiner B, Falkow S 1988; Penetration ofSalmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol 107: 221–230.
Valentin-Weigand P, Chhatwal GS 1995; Correlation of epithelial invasiveness of group B streptococci with clinical source of isolation. Microb Pathog 19: 83–91.
Simons K, Fuller SD 1985; Cell surface polarity in epithelia. Annu Rev Cell Biol 1: 243–288.
Bagg J, Poxton, I-R. Weir, DM, Ross, PW 1982; Binding of type-III group B streptococci to buccal epithelial cells. J Med Microbiol 15: 363–372
Teti G, Teti G, Tomasello F, Chiofalo MS, Orefici G, Mastroeni P 1987; Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect Immun 55: 3057–3064.
Sellin M, Håkaussou S, Norgren M 1995; Phase-shift of polysaccharide capsule expression in group B streptococci, type III. Microb Pathog 18: 401–415.
Marques MB, Kasper DL, Pangburn MK, Wessels MR 1992; Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun 60: 3986–3993.
Townsend GC, Scheld WM 1995; Microbe-endothelial interactions in blood-brain barrier permeability during bacterial meningitis. ASM News 61: 294–298.
Acknowledgements
The authors thank Ingegerd Alriksson for devoted and inspired technical assistance throughout the course of these studies. We also wish to thank Karin Roberg for technical assistance with the electron microscopy studies. The kind gift of GBS reference strains from Stellan Håkansson is gratefully acknowledged.
Author information
Authors and Affiliations
Additional information
Supported by grants from Örebro County Council. King Gustaf Vth 80-Year Foundation, the Swedish Rheumatism Association, and the Börje Dahlin's Foundation.
Rights and permissions
About this article
Cite this article
Källman, J., Kihlström, E. Penetration of Group B Streptococci through Polarized Madin-Darby Canine Kidney Cells. Pediatr Res 42, 799–804 (1997). https://doi.org/10.1203/00006450-199712000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199712000-00014
This article is cited by
-
Group B Streptococcus: global incidence and vaccine development
Nature Reviews Microbiology (2006)