Abstract
The deposition efficiency of three methods of aerosol delivery of salbutamol into lungs of ventilated rabbits was compared: 1) metered dose inhaler (MDI) with holding chamber (HC), 2) jet nebulizer (JN), and 3) ultrasonic (US) nebulizer. The latter system was tested using two different sized medication reservoirs, a large (20 mL) cup (US20) and a small (10 mL) cup (US10). After delivery of technetium-99m-labeled sulbutamol aerosol, deposition in the lungs, trachea, and ventilator circuit were estimated by a γ counter. Total pulmonary deposition [mean(SEM)] as a percentage of the prescribed drug was: MDI + HC 0.22(0.05)%; JN 0.48(0.05)%; US20 0.90(0.13)%; US10 3.05(0.49%)%. Only the deposition from the US10 was statistically significantly higher than the other modes (p < 0.05). Dynamic scintigraphy showed that, among the nebulizers, the US10 continued to deliver medication for longer than either the JN or the US20. We conclude that the US10 appears to be more efficient in delivering aerosol to the lung in this rabbit model and merits further evaluation for clinical efficiency.
Similar content being viewed by others
Main
Use of bronchodilators and steroids in newborns and infants with bronchopulmonary dysplasia or bronchiolitis may improve both lung function and clinical status(1–9). Delivery by the aerosol route rather than by the systemic route would minimize drug delivery to other sites, and thereby potentially reduce systemic side effects. Aerosols used in newborns can be generated by either MDI or the JN or US nebulizers(10). The most commonly used modality is the JN, widely used for therapy in both ventilated and nonventilated newborns. However, JNs have been previously shown to deliver a maximum of 2% of the prescribed drug into the lungs of ventilated infants or small lung models(10–14). Even JNs that minimize losses by generating submicronic aerosols still result in low pulmonary deposition(12, 14). To overcome this inefficient delivery, both the MDI with spacer(11, 15) and the US nebulizer have been suggested as alternative aerosol delivery systems for intubated infants. To our knowledge, however, no comparison between the three modalities in the same experimental model is available.
The present study describes the delivery of aerosolized salbutamol to an in vivo ventilated rabbit model to compare the efficiencies of all three modalities-MDI and JN and US nebulizers. Using radiolabeled aerosols, comparisons were made of pulmonary deposition and distribution, airway deposition, and losses in the ventilator circuit.
METHODS
Animal model. A total of 31 healthy white New Zealand rabbits were studied (average birth weight, 3 kg). The study protocol was approved by the McMaster University Animal Ethics Committee. Animals were anesthetized using intramuscular ketamine (40 mg/kg) and xylazine (5 mg/kg). A tracheostomy was performed, and a 3.5-mm endotracheal tube was inserted into the trachea through the tracheostomy for a distance of 2.5 cm. The trachea was tied tightly around the endotracheal tube to prevent leak of either air or aerosol. The animal was then established on mechanical ventilation with either a Siemens Servo 300 (Siemens Elema AB, Sweden) or Bournes BP200 (Bear Medical Systems, Riverside, CA) ventilator. In all the animals, the ventilator settings were maintained unchanged throughout the experiment: peak inflation pressure 12 cm water, positive end-expiratory pressure 2 cm water, rate 30/min, inspiratory time 0.5 s, and 100% oxygen. These ventilator settings had been shown in preliminary studies to provide a tidal volume of 7-10 mL/kg and appropriate ventilation resulting in an arterial Pco2 of 35-40 mm Hg. The inspired gas was warmed and humidified with the gas temperature maintained at 35 °C at the level of the endotracheal tube. A paper filter (Pall Biomedical, FaJardo, Puerto Rico) was connected to the expiratory limb of the ventilator circuit to prevent contamination of the ventilator and the atmosphere by the radioactivity. After institution of mechanical ventilation, the animal was paralyzed using pancuronium (100 μg/kg).
Radiolabeling and calibration of salbutamol formulations. Labeling of salbutamol respirator solution used for nebulization was performed by mixing the prescribed drug dose with 3 mL of 99mTc-labeled HSA (1.6 mg HSA/mL) in saline (approximately 1 mCi/mL). 99mTc-labeled HSA is a nonabsorbable tracer which, when nebulized as a mixture with salbutamol, is released from the nebulizer with a 1:1 ratio between drug and radioactivity(16). Labeling of salbutamol MDI was performed using a variation of the technique described by Summer et al.(17). Briefly, technetium pertechnetate (99mTcO4-) was extracted from saline using methyl ethyl ketone. The solution was then placed in an empty metal canister (Glaxo, Toronto, Ont), and the methyl ethyl ketone was evaporated under a stream of nitrogen. A previously frozen (-70 °C) canister of Ventolin® (Glaxo) was cut open, and the contents immediately poured into the empty metal canister containing the radioactivity. This canister was then sealed with a Neotechnic 356 metering valve (Glaxo) using a Socage Aerosol Crimper, model 721.11 (BLM, Greenwich, CT), and placed in an US bath for 5 min. The labeled MDI was allowed to reach room temperature before calibration and use. Each canister used was assayed for the total amount of radioactivity before administration to the rabbits. The total weight was also obtained to ensure that no leakage of propellent had occurred. After the release of five priming doses from the MDI, calibration of the MDI dose was carried out by assaying the radioactivity released from five individual puffs, each actuated onto a separate cotton ball. Only canisters producing consistent doses per actuation with less than 15% variability were accepted for use in vivo. The average of these five actuations in microcuries was used as dose per actuation in the subsequent animal studies. Corrections were made on all the measurements to account for decay of isotope between manufacture of the labeled MDI and quantification of lung and tissue radioactivity after delivery.
Delivery of salbutamol aerosol. The aerosol delivery devices were connected to the ventilator circuit as shown in Figure 1. The animals were divided into four groups. Group 1 (n = 7) received 99mTc-labeled salbutamol from the MDI (approximately 100 μCi/puff) with the aid of a 145-mL volume aerosol holding chamber (MV15 Aerochamber®, Trudell Inc., Canada). As is our standard practice in the Neonatal Intensive Care Unit, immediately before the administration of the medication, the holding chamber was inserted between the T-piece of the ventilator circuit and the endotracheal tube. The use of an in-line holding chamber has been shown to be beneficial for the delivery of MDI aerosols to ventilated patients(18), enhancing delivery by slowing the forward velocity of the aerosol. In accordance with our usual clinical practice, priming of the Aerochamber® with a number of aerosol doses was not performed. However, the MV15 Aerochambers® were washed and air-dried before use (again to comply with our current clinical practice) to reduce the static charge on the walls(19). A total of five puffs (100 μg of salbutamol/puff) of the aerosol were delivered, each individually actuated at end-expiration into the holding chamber and inhaled separately. The time interval between each puff was 1 min. The holding chamber was removed from the circuit 2 min after completion of the delivery of the aerosol.
Group 2 (n = 11) was given the radiolabeled HSA salbutamol solution from a neonatal JN (Flo Thru®, Hudson RCI, Temecuha, CA), operated with pure oxygen at a flow rate of 6 L/min as recommended by the manufacturer. The gas flow rate from the ventilator was reduced accordingly so as to maintain the same total circuit flow rate and ventilation pressures, as otherwise deposition may be altered.
Both group 3 (n = 6) and group 4 (n = 7) received the radiolabeled HSA salbutamol solution from an US nebulizer (Siemens Electronic Inc®., Sweden) with two different sized medication cups. In this nebulizer, the coupling fluid to the transducer is located in an inaccessible sealed compartment under the medication cup. In group 3, the medication cup was the standard nebulizer reservoir with a volume of 20 mL (US20). In group 4, the medication cup had a volume of 10 mL and a tapered bottom (US10). This small medication cup was placed inside the standard 20-mL nebulizer reservoir to which 10 mL of water were added to act as a second coupling solution to the transducer. As no additional gas flow was required, there was no need for any adjustment of the ventilator gas flow rate during nebulization.
As is the standard practice in our Neonatal Unit, the JN and US nebulizers were placed in line with the inspiratory limb of the ventilator circuit, 20 cm away from the attachment of the endotracheal tube Y-piece (Fig. 1). Each dose of medication consisted of 0.1 mL/kg salbutamol respirator solution (100 μg/kg salbutamol) diluted with 3 mL of 99mTc-labeled HSA (activity approximately 1 mCi/mL). Each animal received the nebulized medication for 20 min.
γ Scintigraphy of the lungs. The imaging of the rabbits was performed immediately after the administration of the MDI puffs. As there were a total of five puffs given at intervals of 1 min, totaling 5 min, the scan was performed at 5 min. Dynamic scintigraphy was carried out on animals in groups 2 (JN), 3 (US20), and 4 (US10) during nebulization. The lungs were imaged continuously by a large field of view γ camera (model 410 LFOV, Ohio Nuclear) during nebulization. Twenty frames at 1-min/frame were obtained. A time-activity curve was plotted expressing the amount of radioactivity accumulating in the lung from each frame, as a percentage of the maximum lung activity measured during the last frame.
Estimation of pulmonary deposition and distribution of salbutamol. After delivery of the medication, the animals were killed with i.v. pentobarbitone, and the lungs were excised. The trachea was divided from the main bronchi just above the carina. Each lobe of the lungs was dissected out from the lobar bronchus and, by eye, divided into approximately equally weighed peripheral (subpleural) and central (hilar) portions. The radioactivity in the endotracheal tube, the trachea, the carina region, which consisted of the main and lobar bronchi, and each lung piece was measured by a γ counter (Minaxi Auto-Gamma 5000, Canada), corrected for isotope decay and converted to micrograms of drug using calibration factors. The amounts of radioactivity accumulating in the 20-cm inspiratory tubing, the T-connector, the endotracheal tube connector, as well as the residual radioactivity in the nebulizer at the end of nebulization, were measured in a dose calibrator (Capintec® Inc., Pittsburgh, PA). The activity in the expiratory ventilator tubing and the filter, which were too large to be put into the dose calibrator, was measured by the γ camera.
In JN, US20, and US10 (i.e. groups 2, 3, 4) deposition was also expressed as the percentage of the initial dose of radioactivity placed in either the nebulizer reservoir or in the MDI canister. To demonstrate the distribution of the aerosol in the various lung regions, deposition in each region was calculated from the radioactivity detected in each gram of lung tissue expressed as the percentage of total lung radioactivity.
Estimation of particle size of salbutamol aerosol. The particle size distributions of the salbutamol aerosols from each of the ventilator setups were measured using an 8-stage Non-Viable Anderson Cascade Impactor, operated at 28.3 L/min. Six repeat sizings were performed for each delivery system with the aerosols sampled at the exit of each of the systems and also at the exit of a 3.5-mm endotracheal tube.
Five doses from the MDI + MV15 were actuated into the impactor, one dose every 30 s. Aerosols from the nebulizers were sampled for 30 s to avoid excessive wetting of the impactor stages. The stages of the impactor were assayed for salbutamol using UV spectroscopy at 276 nm (Hitachi Spectrophotometer, model 100-60). The results were plotted on log-probability paper to obtain a mass cumulative distribution curve. The MMAD and geometric SD for each aerosol were determined from these plots. These parameters are universally used to characterize an aerosol. An aerosol with a MMAD < 3 μm has a high probability for depositing in the lungs; aerosols between 1 and 5 μm in size are considered to be within the respirable range.
Statistics. All values are presented as mean (SEM). Comparisons between two groups were made using 2-tailed unpaired t test or Mann-Whitney rank sum test where appropriate. For comparison of repeated paired measurements in the same animals, the paired t test or Wilcoxon signed rank test was used. Comparison among multiple groups was made using one-way ANOVA, followed by the Student-Newman-Keuls test for pairwise multiple comparisons where appropriate.
RESULTS
Particle size distribution. Particle size distribution of the aerosols is given in Table 1. Aerosols delivered by all the systems contained fine particles that were well within the respirable range (MMAD 1-5 μm). Among the systems, the US nebulizer produced the largest particles, whereas particles generated by the JN had the smallest MMAD (p < 0.001, ANOVA). Particle size was reduced for all systems at the exit of the endotracheal tube (p < 0.02, paired t test).
Animals. The four groups of animals were of similar body weight. These figures, in mean(SEM), were as follows: for MDI + HC, 2861(83) g; JN, 2966(81) g; US20, 2765(81) g; US10, 2803(39) g.
Pulmonary deposition of radioactive aerosols. The amount of pulmonary deposition in all four groups, when expressed as the percentage of the prescribed dose delivered into the ventilator circuit, was significantly different among the groups (p < 0.001, one-way ANOVA), being: MDI + HC 0.22(0.05)%; JN 1.13(0.09)%; US20 3.34(0.79)%; US10 5.29(0.77)%. Thereafter, pairwise multiple comparisons by the Student-Newman-Keuls method showed a progressive increase in the amount of deposition from MDI + HC (group 1) through to US10 (group 4) (i.e. HC < JN < US20 < US10, p < 0.05). However, when expressed as the percentage of the initial prescribed dose, only the pulmonary deposition of the US10 group was significantly greater (MDI + HC 0.22(0.05)%; JN 0.48(0.05)%; US20 0.90(0.13)%; US10 3.05(0.49)%, p < 0.001). The differences between the first three groups did not reach statistical significance.
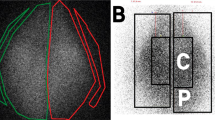
Regional distribution of aerosol in the lung (Figs. 2 and 3). The distribution of aerosol in various regions of the lungs was determined by calculating the amount of radioactivity deposited in each gram of lung tissue, which was then expressed as the percentage of total radioactivity in both excised lungs. There was no significant difference in deposition between the right and left lungs for all four groups as shown in Figure 2. Distribution among the lobes (as a percent of total radioactivity) was uniform in both MDI + HC and JN with no significant differences noted in the amount of lobar deposition. In the US20 and US10 groups, deposition in the anterior azygous lobe was significantly greater than that in the right anterior, posterior azygous, and the right posterior lobes in both groups of animals [US20: 16.3(3.1)%/g versus 7.5(0.9)%/g versus 5.8(0.9)%/g versus 7.3(1.6)%/g, ANOVA: p = 0.004; US10: 15.8(2.8)%/g versus 7.8(1.2)%/g versus 5.9(0.8)%/g versus 5.2(1.2)%/g, ANOVA: p = 0.002, Student-Newman-Keul test: p < 0.05 for both groups]. Distribution between the peripheral and central lung regions is shown in Figure 3. There was a tendency for more aerosol to deposit in the central parts of each lobe and also in both lungs. However, the difference was of statistical significance only in the left posterior lobe of animals in US10 group (central versus peripheral (median): 11.4%/g versus 7.8%/g, p = 0.031, Wilcoxon signed rank test).
Deposition in the airway and ventilator circuit. Most of the radioactivity from the MDI (Table 2) [77.23(5.07)%] remained in the holding chamber, whereas for the other three groups, over 70% of the medication was recovered from the exhalation tubings and filters. There was also considerable deposition (9-16%) in the 20-cm inspiratory tubing distal to the nebulizer. Deposition in the T-connectors were similar for all delivery systems, except for the MDI, where it was much lower. Losses in the carina region of the trachea were similar in all groups, although US20 and US10 animals had significantly greater deposition in the trachea than the other two groups (p < 0.001). The differences in deposition in the trachea and carina, however, did not affect the comparison of lung deposition, because the latter was estimated after removal of the trachea and the carina.
γ Scintigraphy dynamic study (Fig. 4). Data plotted were normalized to the maximum radioactivity (100%) measured in each animal, and this figure constitutes the y axis. The y axis is not a total dose, but rather it is a percentage of the total radioactive dose received. The curves show that the accumulation of radioactivity in the lungs of animals in JN and US20 groups was sinusoidal with a later linear portion. There was an initial period (5 min) of rapid accumulation, followed by a 10-min period during which the rate of deposition decreased. The curves suggested that 80% of the total lung dose of the radiolabeled aerosol delivered by both JN and US20 was deposited in the first 10 min, with only minimal additional deposition during the latter half of nebulization. Over the 20 min recording time, the accumulation curve for US10 animals appeared to be linear, indicating that the deposition of aerosol from the US10 continued at a constant rate almost until the end of the experiment. At 10 min, only about 60% of the total final lung dose was deposited and accumulation of radioactivity continued at a constant rate until approximately 17 min.
DISCUSSION
Little data relevant to aerosol delivery in human newborns exists for any delivery system. Only three studies have directly examined lung deposition in human newborns. Two used an extrapolation from absorbed cromoglycate(12, 15), and one relied upon radioisotope measures(20). To our knowledge no data directly comparing three aerosol delivery modalities-MDI, JN, and US nebulizer has been reported in either human infants or in animal models. The current study showed that of the three delivery modalities, the US nebulizer, using a small medication reservoir, exhibited the highest efficiency for depositing aerosol to the lungs of the mechanically ventilated rabbits. All devices, including the flow rate settings for the JN, were operated according to manufacturer's recommendations.
Previous studies assessing the delivery efficiencies of the MDI and a variety of JNs consistently show relatively low lung deposition values of the range between 0.5 to a maximum of 2% of the prescribed dose. In model studies, these values were usually calculated as deposition beyond the tip of an endotracheal tube, where assumptions were made that this was equivalent to lung delivery. Deposition in the lungs at the bronchiolar level would presumably have been a fraction of this dose, as our study and others show that deposition at the trachea is considerable and exceed that in the lung parenchyma(21). Previous experience with US nebulizers have not demonstrated superiority of this device over the MDI or JN. Evaluation of an US nebulizer with filter test lungs, and in human infants by Cameron et al.(11) and Grigg et al.(15), showed a pulmonary deposition of 0.6 and 1.3% of the initial reservoir dose, respectively. Several maneuvers have been adopted by previous workers to enhance lung deposition. The largest deposition by JN was reported by Cameron et al.(11), who found that by using a relatively high inflation pressure (30 cm H2O) and long inspiration time (1 s), 2.8% of the aerosol released was deposited in the lungs of rabbits. O'Callaghan et al.(21) and Rozycki et al.(22) used the MDI to deliver aerosolized steroid and were able to deposit 1.2 and 1.32% of the actuated dose in rabbit lungs and filters, respectively. In both studies, the MDI was actuated into a collapsible spacer, and the aerosol was “bagged” into the lung or lung models. Using a similar method, Grigg et al.(15) was able to deliver 1.7% of a dose of sodium cromoglycate to the lungs of babies.
Difficulties in delivering aerosol through a neonatal ventilator circuit are at least partly due to loss of aerosol to the circuit or the delivery device. Our data on the fractional deposition showed about 80% of the aerosol delivered by the MDI was retained in the holding chamber. In the nebulized groups, a similar proportion escaped into the expiratory limb of the ventilator tubings. Hence only a small portion of the aerosol generated is available for inhalation.
Our findings on pulmonary deposition concur with previous studies(13–15, 23) in that only a small proportion of the prescribed dose of aerosol was deposited in the lungs of the animals by either MDI or JN. Using the unmodified US nebulizer with a large medication reservoir (US20), pulmonary deposition was not significantly greater, being 0.9% of the initial reservoir dose or 3.34% of the nebulized dose). However, a significant 3-4-fold increase in pulmonary deposition was observed when the US nebulizer was used together with the small medication reservoir (US10). The small reservoir might have enhanced aerosol delivery and hence deposition by a number of mechanisms. Dynamic γ scintigraphy showed that, when the small reservoir was being used, accumulation of radioactivity in the animals' lungs continued over a significantly longer period of time than that in the other nebulized groups. The tapered bottom of the small reservoir might have resulted in a smaller “dead volume,” and more complete nebulization of the medication(24). However, the drug solution inside the small reservior cup was completely surrounded by the coupling fluid, which would have resulted in better transmission of US energy and a greater aerosol output. The potential clinical relevance, if any, of the more prolonged delivery of aerosol from the US10 to the faster time to maximal percent deposition by the other devices is unknown.
Few studies have examined the distribution of aerosol in the lungs. Using γ scintigraphy, Cameron et al.(11) showed good peripheral distribution of radioactivity in the lungs of intubated rabbits that were given radiolabeled aerosol by a JN. Everard et al.(23) used the same technique on ventilated rabbits and demonstrated uniform deposition in the lung lobes of radiolabeled aerosol delivered by the MDI. From our results in all groups of animals studied, there was a tendency for the aerosol to concentrate at the central lung regions, more so when the US nebulizer was used. However, a substantial proportion could be delivered to the periphery. Distribution between the lung lobes was fairly even, although in animals receiving the aerosol from the US nebulizer with both sizes of medication reservoir, deposition in the anterior azygous lobe tended to be greater than that in the other lobes. The droplet size distribution showed that the aerosol produced by the US nebulizer had a MMAD greater than that by the JN, but only slightly larger than that of the MDI and MV15 spacer. With all devices, particle size was reduced at the exit of the ETT. All these aerosols, however, were fine aerosols with over 90% of their droplets within the size range considered respirable, and should, theoretically, be able to penetrate into the lung periphery. However, the flow pattern of the inspired gas, along with the short inspiratory time, might have caused increased deposition in the trachea and central airways for all three modalities(25).
This study had some methodologic differences related to the labeling of the compounds used to tag the lung. Salbutamol-nebulized solution was labeled with 99mTc-HSA. HSA is a nonabsorbable tracer, whereas salbutamol delivered by MDI was labeled with pertechnetate (99mTc-O4), which is absorbed into the circulation with a t1/2 of 10-15 min in rabbits and in man. Thus as described in “Methods” the imaging protocol was necessarily different within two areas of the study. However, this difference in uptake of the tracer should not have influenced the outcomes in this study. In addition, in a recent study to examine deposition in infants with bronchopulmonary dysplasia using MDI and the pertechnetate label, no thyroid deposition was visible(20), further supporting our methodology. This is because in the case of significant absorption, this would result in thyroid uptake and thus visibility. Moreover, the scanning of the MDI group was performed within 5 min of the first puff and immediately after the last puff. This time interval we feel is unlikely to have been long enough for significant loss of radioactivity due to absorption from the lung in this animal preparation without acute lung injury. Furthermore, as dynamic imaging of the lung was performed on the animals who received HSA only by inhalation, the concern regarding a decreased lung count due to absorption from the lung in the MDI group should be unfounded. Furthermore, data from this latter group was corrected in the effective half-life of the isotope in the lung (t1/2 = 15 min).
In conclusion, we compared aerosol delivery by MDI and holding chamber, JN, and an US nebulizer with two sizes of medication reservoir under identical experimental conditions, and showed that the US nebulizer incorporated with a small medication reservoir delivered a significantly greater amount of aerosol to the lungs of ventilated rabbits than did all the other devices. Our findings, however, may be specific to the models of the devices used in the study and may not be generalizable to devices of a different make. Animal studies investigating aerosol delivery are limited by differences in the branching pattern of airways between animals and humans. Aerosol delivery is affected by the presence of lung pathology(26) and the different types of lung diseases, in particular airway and parenchymal diseases, which would alter mechanical ventilation parameters and therefore aerosol delivery. Thus our findings on healthy animal lungs, with different ventilation of peripheral airways, might not be able to be easily extrapolated to human infants with marked inhomogeneity of ventilation, who require aerosol therapy. Despite these limitations, the superior performance of the US nebulizer with the small reservoir is encouraging and deserves further exploration with a randomized clinical study.
Abbreviations
- MDI:
-
metered dose inhaler
- JN:
-
jet nebulizer
- US:
-
ultrasonic
- HSA:
-
human serum albumin
- MMAD:
-
mass median aerodynamic diamete
References
LaForce WR, Brudno DS 1993 Controlled trial of beclomethasone dipropionate by nebulization in oxygen- and ventilator-dependent infants. J Pediatr 122: 285–288.
Yuksel B, Greenough A, Maconochie I 1990 Effective bronchodilator treatment by a simple spacer device for wheezy premature infants. Arch Dis Child 65: 782–785.
Pfenninger J, Aebi C 1993 Respiratory response to salbutamol (albuterol) in ventilator-dependent infants with chronic lung disease: pressurized aerosol delivery versus intravenous injection. Intensive Care Med 19: 251–255.
Cabal LA, Larrazabal C, Ramanathan R, Durand M, Lewis D, Siassi B, Hodgman J 1987 Effects of metaproterenol on pulmonary mechanics, oxygenation, and ventilation in infants with chronic lung disease. J Pediatr 110: 116–119.
Wilkie RA, Bryan MH 1987 Effect of bronchodilators on airway resistance in ventilator-dependent neonates with chronic lung disease. J Pediatr 111: 278–282.
Rotschild A, Solimano A, Puterman M, Smyth J, Sharma A, Albersheim S 1989 Increased compliance in response to salbutamol in premature infants with developing bronchopulmonary dysplasia. J Pediatr 115: 984–991.
Denjean A, Guimaraes H, Migdal M, Miramand JL, Dehan M, Gaultier C 1992 Dose-related bronchodilator response to aerosolized salbutamol (albuterol) in ventilator-dependent premature infants. J Pediatr 120: 974–979.
Kraemer R, Birrer P, Modelska K, Casaulta Aebischer C, Schöni MH 1992 A new baby-spacer device for aerosolized bronchodilator administration in infants with bronchopulmonary disease. Eur J Pediatr 151: 57–60.
Kao LC, Warburton D, Platzker ACG, Keens TG 1984 Effect of isoproterenol inhalation on airway resistance in chronic bronchopulmonary dysplasia. Pediatrics 73: 509–514.
Arnon S, Grigg J, Nikander K, Silverman M 1992 Delivery of micronized budesonide suspension by metered dose inhaler and jet nebulizer into neonatal ventilator circuit. Pediatr Pulmonol 13: 172–175.
Cameron D, Arnot R, Clay M, Silverman M 1991 Aerosol delivery in neonatal ventilator circuits: a rabbit lung model. Pediatr Pulmonol 10: 208–213.
Watterberg KL, Clark AR, Kelly HW, Murphy S 1991 Delivery of aerosolized medication to intubated babies. Pediatr Pulmonol 10: 136–141.
Cameron D, Clay M, Silverman M 1990 Evaluation of nebulizers for use in neonatal ventilator circuits. Crit Care Med 18: 866–870.
Flavin M, MacDonald M, Dolovich M, Coates G, O'Brodovich H 1986 Aerosol delivery to the rabbit lung with an infant ventilator. Pediatr Pulmonol 2: 35–39.
Grigg J, Arnon S, Jones T, Clarke A, Silverman M 1992 Delivery of therapeutic aerosols to intubated infants. Arch Dis Child 67: 25–30.
Fuller HD, Dolovich M, Chambers C, Newhouse MT 1992 Aerosol delivery during mechanical ventilation: a predictive in vivo lung model. J Aerosol Med 5: 251–259.
Summer QA, Hollingsworth A, Clark AR, Fleming J, Holgate ST 1990 The preparation of a radiolabelled aerosol of nedocromil sodium for administration by metered-dose inhaler that accurately preserves particle size distribution of the drug. Drug Invest 2: 90–98.
Manthouss CA, Hall JB, Schmidt GA, Wood LDH 1993 Metered-dose inhaler versus nebulized albuterol in mechanically ventilated patients. Am Rev Respir Dis 148: 1567–70.
Dolovich M, Mitchell JP, Nagel MW 1995 Comparison of performance of aluminum with plastic holding chambers with four drugs used in inhalation therapy. Eur Respir J 8( suppl 19): 201s.
Fok TF, Monkman S, Dolovich M, Gray S, Coates G, Paes B, Rashid F, Newhouse M, Kirpalani H 1996 Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Ped Pulmonol 21: 301–309.
O'Callaghan C, Hardy J, Stammers J, Stephenson TJ, Hull D 1992 Evaluation of techniques for delivery of steroids to lungs of neonates using a rabbit model. Arch Dis Child 67: 20–24.
Rozycki HJ, Bryon PR, Dailey K, Gutcher GR 1991 Evaluation of a system for the delivery of inhaled beclomethasone dipropionate to intubated neonates. Dev Pharmacol Ther 16: 65–70.
Everard ML, Stammers J, Hardy JG, Milner AD 1992 New aerosol delivery system for neonatal ventilator circuits. Arch Dis Child 67: 826–830.
Madsen F, Nielsen NH, Frølund L, Svendsen VG, Weeke B 1987 Bronchial challenge: a small reservoir for the wright jet nebulizer. Bull Eur Physiol Pathol Respir 23: 67–71.
O'Doherty MJ, Thomas SML, Page CJ, Treacher DF, Nunan TO 1992 Delivery of a nebulized aerosol to a lung model during mechanical ventilation. Am Rev Respir Dis 146: 383–388.
Dolovich MB, Sanchis J, Rossman C, Newhouse MT 1976 Aerosol penetrance: a sensitive index of peripheral airway obstruction. J Appl Physiol 40: 468–471.
Acknowledgements
The authors thank Siemens Canada and Siemens Elema AB, Sweden, who provided us with the US nebulizers, and Pat Haslett and Sheryl Fisker for their excellent secretarial help.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fok, TF., Al-Essa, M., Monkman, S. et al. Pulmonary Deposition of Salbutamol Aerosol Delivered by Metered Dose Inhaler, Jet Nebulizer, and Ultrasonic Nebulizer in Mechanically Ventilated Rabbits. Pediatr Res 42, 721–727 (1997). https://doi.org/10.1203/00006450-199711000-00027
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199711000-00027
This article is cited by
-
Development of a High-Dose Infant Air-Jet Dry Powder Inhaler (DPI) with Passive Cyclic Loading of the Formulation
Pharmaceutical Research (2022)
-
Development of an ex vivo respiratory pediatric model of bronchopulmonary dysplasia for aerosol deposition studies
Scientific Reports (2019)
-
Development of a New Technique for the Efficient Delivery of Aerosolized Medications to Infants on Mechanical Ventilation
Pharmaceutical Research (2015)
-
Administration d’aérosols médicamenteux au cours de la ventilation mécanique
Réanimation (2012)