Abstract
In this study, the diagnostic and predictive value of brainstem, middle latency, and cortical auditory evoked responses (BMC-AERs) obtained in the neonatal period in 81 preterm infants was assessed in relation to neurodevelopmental outcome. The preterm infants were neonatally classified according to risk category and gestational age. The BMC-AERs were analyzed with respect to detectability, latencies, and amplitudes as well as derived latency and amplitude measures. At 5 y of age the neurodevelopmental outcome was assessed from neurologic and neuropsychologic evaluations. The results showed that BMC-AER differences mainly correlated with risk category (low risk/high risk) and to some extent with degree of prematurity. In view of these findings the degree of prematurity and the effect of risk category have to be taken into account, when BMC-AERs are applied in the preterm period to predict neurodevelopmental outcome. In this study the BMC-AERs for infants with abnormal neurodevelopmental outcome were scarcely distinguishable from the BMC-AERs for infants with normal neurodevelopmental outcome. Thus far, this and previous reports have indicated that BMC-AERs in preterm infants are useful in maturational studies and with infants showing symptoms related to lesions or dysfunction of the peripheral and/or central auditory system. For predicting neurodevelopmental outcome in preterm infants, BMC-AERs are of limited clinical value.
Similar content being viewed by others
Main
The relatively high short-term and long-term morbidity in preterm infants has encouraged many authors to focus on the determination of neonatal risk factors to improve the prediction of neurodevelopmental outcome(1–3). Several neonatal risk factors and risk scores have been proposed as predictors of neurodevelopmental outcome in preterm infants and/or infants with low birth weight(3, 4). However, these neonatal risk factors and risk scores are of limited clinical value, because a considerable number preterm infants predicted to be at low risk develop neurodevelopmental impairments during infancy or childhood.
Brainstem (ABR), middle latency (MLR), and cortical (ACR) auditory evoked responses (BMC-AERs) primarily reflect the function of the peripheral and central auditory pathways. To a certain extent the BMC-AERs also give information on the integrity of adjacent parts of the CNS. Several experiments have demonstrated selective vulnerability of auditory relay nuclei in the preterm period, especially between 28 and 40 wk GA(5, 6). In the past 15 y several authors have reported on the clinical importance of ABRs in newborn infants. Most of these studies have focused primarily on term infants, in particular on term infants with asphyxia or hyperbilirubinemia and on term infants at risk for hearing loss(7–9). Neonatal ABR abnormalities have been described in infants with perinatal complications(10–12). Some authors have stated that early physiologic indices can be used to predict long-term developmental trends(13). Some studies have concentrated on the predictive value of ABRs in relation to neurodevelopmental outcome or later language skills in term infants(13, 14). Only few authors have reported on ABR findings related to neurodevelopmental outcome in preterm infants(15–17). Majnemer et al.(14) found evidence for the usefulness of the ABR as a diagnostic test for high risk neonates. Cox et al.(15) put forward that early ABR may predict long-term neurobehavioral development in low birth weight infants. Salamy and Eldredge(16) reported that infants with neurologic signs or demonstrable brain anomalies were four to five times more likely to exhibit deviant ABRs. They also reported that the synergistic effects of selected predictor variables further increased the risk associated with abnormal ABRs. On the other hand, Beverly et al.(17) found that neither flash VERs nor ABRs provide a good prognostic indicator for neurodevelopmental outcome. Only a few reports have focused on the predictive value of the MLR and ACR for neurodevelopmental outcome in newborn infants(18). To our knowledge, BMC-AER abnormalities are not evaluated as a neonatal risk factor in preterm infants.
In the present study the diagnostic and predictive value of BMC-AERs obtained in preterm infants in the neonatal period is assessed in relation to the long-term neurodevelopmental outcome. First, we studied the differences in (cumulative) detectability of the various BMC-AER components obtained in the preterm period between low risk and high risk preterm infants, early and late preterm infants, and infants with and without a normal neurologic outcome at 5-7 y of age. We also studied the influence of the degree of prematurity on BMC-AERs obtained at 29-32-wk CA and at 33-35-wk CA, for early low risk infants and early high risk infants with neurologic/neuropsychologic abnormalities at age 5-7 y. Furthermore, we analyzed the BMC-AER differences at 33-35-wk CA between early and late low risk preterm infants and between low risk preterm infants with normal and abnormal neurologic/neuropsychologic outcome at age 5-7 y. Finally, the individual BMC-AER results for infants with an abnormal neurodevelopmental outcome at age 5-7 y were analyzed. This study has been approved by the Ethics Committee of the University Hospital Nijmegen. Informed consent was obtained from all parents of infants in this study.
METHODS
Patients. Eighty-one randomly selected preterm infants (GA 25-34 wk) were included in this prospective study. This group consisted of inborn and outborn infants who were admitted to the Neonatal Intensive Care Unit of the University Hospital Nijmegen between 1983 and 1985. Infants with dysgenetic brain lesions, major congenital anomalies, or well defined clinical syndromes were excluded from the study.
In the neonatal period the infants were classified as high risk or low risk according to the semiquantitative NNI. The NNI assessment was performed in the first 2 wk after birth. The NNI is based on four items: 1) clinical neurologic examination, 2) echoencephalography, 3) arterial or capillary blood pH, and 4) Apgar score. Based on the NNI, 65 of the 81 preterm infants were classified as low risk and 16 as high risk (i.e. having one or more of the four NNI high risk criteria). Five of the 65 low risk infants and seven of the 16 high risk infants died in the neonatal period. Forty-four of the surviving low risk infants (73%), and all of the nine surviving high risk infants (100%) had a complete follow-up. The other infants were not available to follow-up because of migration or withdrawal by the parents. The patient characteristics and methods to assess long-term neurodevelopmental outcome are reported in a companion study(2).
BMC-AER recordings. BMC-AERs were obtained every 2 wk during the preterm period, at 40-wk CA, 52-wk CA, and at 5-7 y of age using a Nicolet CA 1000 or Nicolet Pathfinder. Stimulation parameters and other technical data are given in Table 1(19–21). Based on previous studies performed by our research group we have selected those BMC-AER parameters that were determined to be of clinical relevance in preterm infants(22–24). These parameters were: the latencies and amplitudes of ipsilateral ABR components I, II, III, and V, IPLs III-I, V-III, V-I, IPL ratio V-III/III-I, and amplitude ratio V/I. For the the contralateral derivation these ABR parameters were: latencies and amplitudes of contralateral ABR components IIc and Vc and IPL Vc-IIc. For the MLR latencies and amplitudes of components P0 and Na, IPL Na-P0 and PPA P0-Na were analyzed. The latencies and amplitudes of ACR components Na, PbP1, P2p (p = preterm wave form), N2p, IPL N2p-P2p, and PPA P2p-N2p were also analyzed, but at 33-35 wk only the latencies Na and N2p were considered. Detectability of individual BMC-AER components was determined for infants with registrations obtained both during 30-34-wk CA and 35-41-wk CA. The last registration during 30-34-wk CA and the first registration during 35-41-wk CA were considered. Differences in detectability (30-34-wk CA) or cumulative detectability (30-34- and/or 35-41-wk CA) of BMC-AER components between several subgroups were studied. At the age of 5-7 y, a follow-up investigation was performed consisting of a neurophysiologic, neurologic, and neuropsychologic evaluation.
Based on the NNI and the neurologic evaluation at 5-7 y of age, four subgroups were determined: 1) low risk infants without major neurologic abnormalities, 2) low risk infants with major neurologic abnormalities, 3) high risk infants without major neurologic abnormalities, 4) high risk infants with major neurologic abnormalities. Based on the NNI and the neuropsychologic evaluation at 5-7 y of age, four analogous subgroups were determined.
Statistics. The statistical analyses for examining differences between low risk and high risk preterm infants, between early (25-30-wk GA) and late (31-34-wk GA) preterm infants and between normal and abnormal neurologic, respectively, neuropsychologic outcome at 5-7 y, for the (cumulative) detectability of the various BMC-AER components, were performed using Fisher's exact test for 2 × 2 tables. Before performing further statistical tests, the distributions of the different BMC-AER parameters were studied. Although sometimes relatively large values of skewness occurred, no important deviations from Gaussian distributions could be established. In those cases with the largest values of skewness we performed, next to the t tests, also nonparametric tests (Kruskal-Wallis test). No differences between these two methods were found. Because of uniformity with respect to the statistical approach, only the results according to the t tests are presented. The BMC-AER differences between the group of early low risk infants and early high risk infants with neurologic or neuropsychologic abnormalities at age 5-7 y were analyzed for the recordings at 30 wk (or 29-31 wk) and 34 wk (or 33-35 wk) for averaged left-right observations (including incomplete observations) with two-sample t tests. To analyze the BMC-AER differences at 34-wk CA (33-35-wk CA) between early and late low risk preterm infants and between normal and abnormal neurologic or neuropsychologic outcomes, two-way analyses of variance were used for averaged left-right observations (including incomplete observations).
Any difference was considered to be statistically significant if a p value ≤ 0.05 was found. In case 0.05 < p ≤ 0.10 it was said that a trend to differences could be shown. The individual BMC-AER results were assessed using reference values obtained by the authors(19–22). The statistical analyses were carried out using the SAS statistical package(25).
RESULTS
Detectability of BMC-AER components. Table 2 shows the absence of the complete BMC-AER recording in the preterm infants with a normal long-term neurodevelopment, and in the preterm infants with an abnormal long-term neurodevelopment. No important differences with respect to the presence or absence of the complete ABR, MLR, or ACR were found. The majority of the absent ABR, MLR, or ACR recordings were, as could be expected, obtained between 25- and 30-wk CA.
The effect of risk category was assessed by analyzing the differences in (cumulative) detectability between the low risk and high risk subgroups, irrespective of outcome or degree of prematurity (Table 3). The detectability at 30-34-wk CA for ABR components I, III, and Vc, MLR component Na and ACR component Na was significantly higher in the low risk subgroup than in the high risk subgroup for at least one stimulation side and/or derivation. For MLR component Pa and for ACR component P2p the detectability was significantly lower in the low risk subgroup than in the high risk subgroup for one stimulation side and/or derivation. For the cumulative detectability the differences between the high risk and low risk subgroup were smaller than for the detectability at 30-34-wk CA. Generally, the results for detectability and cumulative detectability pointed in the same direction. Because no large differences in (cumulative) detectability between left-sided stimulation and right-sided stimulation (ABR and MLR) and different derivations/electrodes (ACR) were found, we give only the significant differences or trends to differences after left-sided stimulation, with respect to left derivation.
The effect of prematurity on the detectability of BMC-AER components was studied by analyzing the differences in (cumulative) detectability between early low risk and late low risk preterm groups with a normal neurologic outcome. The size of the other early and late subgroups did not permit analogous analyses. For the ABR and ACR no clear differences were found between these subgroups. The detectability of MLR component Pa was significantly lower in the early low risk subgroup with a normal outcome after left-sided stimulation for two derivations.
The relation between the detectability of BMC-AER components and the long-term outcome was determined by analyzing the differences in (cumulative) detectability between the neurologically normal and the neurologically abnormal late low risk group. For ACR component P2p a significantly higher cumulative detectability was found for the left lateral derivation in neurologically normal infants. The size of the other subgroups did not permit analogous analyses.
BMC-AER differences related to neonatal risk category. The significant differences or trends to differences for BMC-AERs obtained at 29-31-wk CA and 33-35-wk CA between early low risk infants and early high risk infants with neurologic or neuropsychologic abnormalities at 5-7 y of age are given in Table 4, A and B.
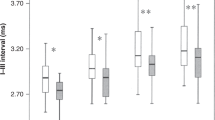
For abnormal neurologic outcomes, the mean ABR latencies 1 and Vc, ABR IPL Vc-IIc, MLR latency P0, and ACR latencies Na (lateral derivation) and P2p (central derivation) obtained between 29- and 31-wk CA were significantly longer in the early high risk infants than in the early low risk infants. For the contralateral derivation the PPA of P0-Na was significantly lower in high risk infants. For abnormal neuropsychologic outcomes, the same holds for ABR latencies I and Vc, and ABR IPL Vc-IIc, MLR latency P0, and ACR latency Na (central derivation).
At 33-35-wk CA, for abnormal neurologic outcomes, significant differences were found between early high risk and low risk infants with respect to ABR IPL V-III, ABR IPL ratio V-III/III-I, and MLR latency P0, with higher means in the early high risk infants. For abnormal neuropsychologic outcomes, significant differences were found for ABR latencies IIc and MLR latency P0, in which early high risk infants also showed higher means than early low risk infants. The MLR IPL Na-P0 was significantly lower in early high risk infants.
Relation between outcome, degree of prematurity and BMC-AER measures. In a two-way analysis of variance, the effect GA on the BMC-AERs obtained between 33- and 35-wk CA, as well as the effect of outcome on these BMC-AERs in low risk infants was evaluated. Generally, the means of the BMC-AER variables of the four subgroups were similar. Averaged over neurologically normal and abnormal, the mean MLR PPA Na-P0 (contralateral) was significantly higher for late preterm infants. Averaged over early and late the mean ACR Na latency was significantly higher in neuropsychologically abnormal infants. In general, the estimated effects of GA were of the same magnitude as the effects of neurodevelopmental outcome. In Table 5 the significant differences or trends to differences are given.
Individual BMC-AERs and abnormal neurodevelopmental outcome. The individual BMC-AER results of high risk and low risk infants with neurologic and/or neuropsychologic abnormalities at 5-7 y of age are tabulated in Table 6 (high risk infants) and Table 7 (low risk infants). Applying our references values, five of the eight surviving high risk infants (63%) and two of 12 low risk infants (17%) showed an abnormal ABR(19–21). The abnormalities consisted of the absence of all components (three high risk infants), one or more components (one low risk infant) or increased (inter-peak) latencies (two high risk infants and one low risk infant). One low risk infant showed the absence of ABR components IIc and Vc at 27-wk CA. However, at this CA level the absence of these components cannot be considered as abnormal. Three surviving high risk (38%) and nine low risk (75%) infants with an abnormal outcome had normal ABRs at the first recording.
For the MLR, six of eight surviving high risk infants (75%) and none of 12 low risk infants (0%) with an abnormal neurodevelopmental outcome had abnormal MLR responses. None of the surviving high risk (0%) and 10 of the low risk (83%) infants had a normal MLR. At this CA level the absence of one or more MLR components in two surviving high risk and two low risk infants cannot be considered as abnormal. The second follow-up MLR registration in these infants showed MLR abnormalities in one of the two surviving high risk infants and none of the two low risk infants.
For the ACR, four of the eight surviving high risk infants (50%) and one of the 12 low risk infants (8%) showed an abnormal ACR response. One of the surviving high risk (13%) and none of the low risk (0%) infants showed a normal ACR. Because of the CA level at time of registration the ACRs of three of the surviving high risk (38%) and 11 of the low risk (92%) infants cannot be considered as abnormal. We also analyzed the AERs of the high risk preterm infants who died in the neonatal period. Two of the seven infants (29%) showed ABR abnormalities, one infant (14%) had a normal ABR and four infants (57%) had one or more absent ABR components, but because of the CA-level at the time of registration this does not necessarily imply abnormality. The same holds for the MLR and the ACR.
DISCUSSION
Changes in BMC-AERs can be related to normal and abnormal functional and/or structural alterations of the auditory system. In preterm infants BMC-AERs reflect the functional and structural maturation of the auditory system. The appearance of BMC-AERs in preterm infants born between 25- and 29-wk CA implies a certain degree of maturation of the auditory system(19–21, 26). Myelination of the auditory pathway is considered to be a requisite for function of the auditory system. Indeed, several studies have shown that myelination of the cochlear nerve and auditory structures in the brainstem starts between 26 and 29 wk CA or even as early as 24 wk CA(27–29). For the majority, the myelination of the auditory brainstem pathways is completed between 40 and 44 wk CA(28, 29). Other factors besides myelination, such as increasing synaptic efficiency, dendritic growth, and the summation, synchronization, and phase-locking capabilities of the auditory system, are also important in determining the central auditory conduction(28, 30, 31). The functional maturation of the auditory pathway is reflected by the changes in detectability, latencies, and amplitudes of BMC-AER components(19–21).
Prematurity predisposes infants to a variety of neurodevelopmental and educational sequelae(15). The early identification of infants who will exhibit long-term neurodevelopmental abnormalities is difficult, and there are frequent false-negative results, i.e. a substantial number of infants classified as low risk will show neurodevelopmental impairments in infancy or childhood. Several neuropathologic studies have demonstrated that, in the preterm period, in particular auditory structures in the brainstem, such as the cochlear nuclei, superior olivary complexes, and inferior colliculi, are vulnerable to perinatal anoxic-ischemic insults. Some authors have suggested that ABRs may predict long-term neurobehavioral development in preterm and low birth weight infants(14–18). However, BMC-AER measures are not thoroughly evaluated as neonatal risk factors(5, 6).
To determine whether BMC-AERs in high risk infants differ from those obtained in low risk infants, we compared the BMC-AER results of high risk and low risk subgroups. For both detectability and cumulative detectability differences were found between low risk and high risk infants for ABR, MLR, and ACR. Generally, the high risk group showed lower (cumulative) detectability rates than the low risk group. Differences between low risk and high risk infants were found also for BMC-AER latencies, IPLs, and amplitude measures (PPA and amplitude ratio). Because the size of some groups was too small, the comparison between low and high risk infants could be analyzed only for the early preterm infants with an abnormal neurologic/neuropsychologic outcome. In general, the latencies and IPLs were longer and the PPAs were smaller in early high risk infants than in early low risk infants. The individual recordings showed also more ABR abnormalities in high risk preterm infants than in low risk infants. Much the same holds for the analysis of the individual MLR and ACR recordings. These longer latencies, longer IPLs, and lower PPAs can be the result of de/dysmyelination or delayed myelination and synaptic evolution of the central auditory pathway(28, 30, 31). Because the BMC-AER differences were generally more clear for recordings obtained at 29-31-wk CA than for recordings obtained at 33-35-wk CA, a delayed myelination seems more likely than a de/dysmyelination(23, 24).
In a previous report we have shown that the detectability of BMC-AER components increase during the neonatal period(22). Generally, in the preterm period we found no clear differences in (cumulative) detectability with respect to the degree of prematurity and neurologic/neuropsychologic outcome. Previously we also reported that preterm birth affects the maturation of BMC-AERs, even in preterm infants with a normal neurodevelopmental outcome at 5 y of age(23, 24). Therefore, the degree of prematurity has to be taken into account when the relation between BMC-AERs and neurodevelopmental outcome in preterm infants is studied. A complex pattern of BMC-AER differences was found between the various preterm subgroups (related to outcome and taking the degree of prematurity into account). The differences consisted not only of longer mean latencies and IPLs for early and abnormal low-risk infants, but concerning some BMC-AER measures also of shorter means. Based on the individual BMC-AER recordings of the preterm infants, no clear distinction could be made between preterm infants with and preterm infants without an abnormal neurodevelopmental outcome. This complex pattern of (generally small) BMC-AER differences might be due, in part, to potentially counteracting (patho) physiologic effects on the maturation of BMC-AERs. Whereas, delayed myelination results in longer latencies and IPLs and lower PPAs, other mechanisms, such as a disturbed synaptic efficacy or reduced dendritic growth, may result in less complex auditory relay centres and, accordingly, to shorter latencies and IPLs. Some of these counteracting effects might be related to prematurity alone, whereas others might be related to pathophysiologic mechanisms or combinations of prematurity and pathophysiologic mechanisms. These opposing effects might result in smaller BMC-AER differences than expected, masking functional and/or structural changes in the auditory system.
This study shows that neonatally obtained BMC-AERs in preterm infants are influenced by risk category (high risk/low risk) as well as degree of prematurity, and that BMC-AERs are, to some extent, also related to neurodevelopmental outcome. However, the effect of risk category is more clear than the effect of the degree of prematurity, or the effect related to neurodevelopmental outcome. These results imply that low risk preterm infants with a later abnormal neurodevelopmental outcome are barely detectable in the neonatal period using BMC-AERs. Because delayed myelination is likely to be an important factor in the BMC-AER differences found between low risk and high risk infants, prenatally emerging cerebral lesions may account for these differences. Recently, Burke and Tannenberg(32) reported that placental infarctions are associated with prenatal cerebral ischemic lesions. These prenatal cerebral ischemic lesions consist primarily of periventricular white matter lesions. These white matter lesions might be associated with disturbed myelination and result in transient BMC-AER abnormalities. Furthermore, it has been suggested that the maturation of the ABR can be faster in growth-retarded newborn infants than in appropriately growing infants. It has been postulated that the faster maturation might be related to raised levels of steroid hormones and catecholamines caused by placental dysfunction. It is known that steroid hormones and catecholamines have a variety of effects on neural maturation(33).
From the present study we conclude that neonatally obtained BMC-AERs in preterm infants cannot be used to generate a simple neonatal risk factor, because there is no strong relationship between BMC-AERs obtained in low risk preterm infants, and later neurodevelopmental outcome. These results, however, do not preclude the use of BMC-AERs as an additional indicator of risk, to strengthen the validity and/or predictive value of (current) neonatal risk scores. Furthermore, BMC-AERs obtained in preterm infants can be useful in maturational studies, in preterm infants at risk for hearing disorders, and in infants clinically suspected of central (or peripheral) dysfunction of the auditory system.
Abbreviations
- ABR:
-
auditory brainstem evoked response
- ACR:
-
auditory cortical evoked response
- BMC-AER:
-
brainstem, middle latency and cortical auditory evoked response
- CA:
-
conceptional age
- GA:
-
gestational age
- IPL:
-
interpeak latency
- MLR:
-
auditory middle latency evoked response
- NNI:
-
neonatal neurologic inventory
- PPA:
-
peak-to-peak amplitude
References
Kitchen WH, Ryan MM, Rickards A, McDougall AB, Billson FA, Keir EH, Naylor FD 1980 A longitudinal study of very low-birthweight infants. IV. An overview of performance at eight years of age. Dev Med Child Neurol 22: 172–188.
Pasman JW, Rotteveel JJ, Maassen B, de Graaf R, Visco Y 1997 Diagnostic and predictive value of auditory evoked responses in preterm infants: I. Patient characteristics and long-term neurodevelopmental outcome. Pediatr Res 42: 665–669.
Scheiner AP, Sexton ME 1991 Prediction of developmental outcome using a perinatal risk inventory. Pediatrics 88: 1135–1143.
Brazy JE, Eckerman CO, Oehler JM, Goldstein RI, O'Rand AM 1991 Nursery neurobiologic risk score: important factors in predicting outcome in very low birthweight infants. J Pediatr 118: 783–792.
Griffiths AD, Laurence KM 1974 The effect of hypoxia and hypoglycemia on the brain of the newborn human infant. Dev Med Child Neurol 16: 308–319.
Leech RW, Alvord EC 1977 Anoxic-ischemic encephalopathy in the human neonatal period: the significance of brainstem involvement. Arch Neurol 34: 109–113.
Guinard C, Fawer CL, Despland PA, Calame A 1989 Auditory brainstem responses and ultrasound changes in a high-risk infants population. Helv Paediatr Acta 43: 377–388.
Durieux Smith A, Picton TW, Bernard P, MacMurray B, Goodman JT 1991 Prognostic validity of brainstem electric response audiometry in infants of a neonatal intensive care unit. Audiology 30: 249–265.
Yang EY, Stuart A, Mencher GT, Mencher LS, Vincer MJ 1993 Auditory brain stem responses to air- and bone-conducted clicks in the audiological assessment of at-risk infants. Ear Hear 14: 175–182.
Yasuhara A, Kinoshita Y, Hori A, Iwase S, Kobayashi Y 1986 Auditory brainstem response in neonates with asphyxia and intracranial haemorrhage. Eur J Pediatr 145: 347–350.
Cycowisz Y, Schmuel M, Freeman S, Wanszelbaum A, Sohmer H 1988 Perinatal hypoxia and auditory brainstem response thresholds: no evidence of permanent hearing loss. Hear Res 33: 239–244.
Murray AD 1988 Newborn auditory brainstem evoked responses (ABRs)longitudinal correlates in the first year. Child Dev 59: 1542–1554.
Karmel BZ, Gardner JM, Zappulla RA, Magnano CL, Brown EG 1988 Brain-stem auditory evoked responses as indicators of early brain insult. Electroencephalogr Clin Neurophysiol 71: 429–442.
Majnemer A, Rosenblatt B, Riley P 1988 Prognostic significance of the auditory brainstem evoked response in high-risk neonates. Dev Med Child Neurol 30: 43–52.
Cox C, Hack M, Aram D, Borawski E 1992 Neonatal auditory brainstem response failure of very low birth weight infants: 8-year outcome. Pediatr Res 31: 68–72.
Salamy A, Eldredge L 1994 Risk for ABR abnormalities in the nursery. Electroencephalogr Clin Neurophysiol 92: 392–395.
Beverley DW, Smith IS, Beesley P, Jones J, Rhodes N 1990 Relationship of cranial ultrasonography, visual and auditory evoked responses with neurodevelopmental outcome. Dev Med Child Neurol 32: 210–222.
Molfese DL 1989 The use of auditory evoked responses recorded from newborn infants to predict later language skills. Birth Defects 25: 47–62.
Rotteveel JJ, de Graaf R, Colon EJ, Stegeman DF, Visco YM 1987 The maturation of the central auditory conduction in preterm infants until three months post term. II. The auditory brainstem responses (ABRs). Hear Res 26: 21–35.
Rotteveel JJ, Stegeman DF, de Graaf R, Colon EJ, Visco YM 1987 The maturation of the central auditory conduction in preterm infants until three months post term. III. The middle latency auditory evoked response (MLR). Hear Res 27: 245–256.
Rotteveel JJ, de Graaf R, Stegeman DF, Colon EJ, Visco YM 1987 The maturation of the central auditory conduction in preterm infants until three months post term. V. The auditory cortical response (ACR). Hear Res 27: 95–110.
Pasman JW, Rotteveel JJ, de Graaf R, Maassen B, Notermans SLH 1991 Detectability of auditory evoked response components in preterm infants. Early Hum Dev 26: 129–141.
Pasman JW, Rotteveel JJ, de Graaf R, Stegeman DF, Visco YM 1992 The effect of preterm birth on brainstem, middle latency and cortical auditory evoked responses (BMC AERs). Early Hum Dev 31: 113–129.
Pasman JW, Rotteveel JJ, de Graaf R, Maassen B, Visco YM 1996 The effects of early and late preterm birth on brainstem and middle-latency auditory evoked responses in children with normal neurodevelopment. J Clin Neurophysiol 13: 234–241.
SAS Institute Inc 1990 SAS User's Guide: Statistics, Version 6 Edition. SAS Institute Inc, Cary, NC, pp 893–892 and 1633–1640.
Ponton CW, Eggermont JJ, Coupland SG, Winkelaar R 1193 The relation between head size and auditory brainstem response (ABR) interpeak latency maturation. J Acoust Soc Am 94: 2194–2158.
Inagaki M, Tomita Y, Takashima S, Ohtani K, Andoh G, Takeshita K 1987 Functional and morphometrical maturation of the brainstem auditory pathway. Brain Dev 9: 597–601.
Moore DR 1985 Postnatal development of the mammalian central auditory system and the neural consequences of auditory deprivation. Acta Otolanryngol Suppl 421: 19–30.
Yakovlev PI, Lecour A 1967 The myelogenetic cycles of regional maturation of the brain. In: Minkowski A (eds) Regional Development of the Brain in Early Life. FA Davis, Philadelphia, pp 3–69.
Javel E 1980 Neurophysiological correlates of auditory maturation. Ann Otol Rhinol Laryngol 89( suppl 74): 103–113.
Romand R 1992 Development of Auditory and Vestibular Systems 2. Elsevier, Amsterdam
Burke CJ, Tannenberg AE 1995 Prenatal brain damage and placental infarction-an autopsy study. Dev Med Child Neurol 37: 555:56562
Amiel Tison C, Pettigrew AG 1991 Adaptive changes in the developing brain during intrauterine stress. Brain Dev 13: 67–76.
Acknowledgements
The authors thank the infants and their parents participating in this study. We thank Bill Sloman for helping with the English version of this manuscript.
Author information
Authors and Affiliations
Additional information
Supported by Grant 28-1940 from “Het Praeventiefonds,” Den Hague. The Netherlands.
Rights and permissions
About this article
Cite this article
Pasman, J., Rotteveel, J., Maassen, B. et al. Diagnostic and Predictive Value of Auditory Evoked Responses in Preterm Infants: II. Auditory Evoked Responses. Pediatr Res 42, 670–677 (1997). https://doi.org/10.1203/00006450-199711000-00020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199711000-00020