Abstract
Despite widespread reports of the vasodilatory actions of nitric oxide(NO), little is known of the relaxant effect of NO on newborn airways or lung parenchymal structures. We studied the effects of inhaled NO at 20, 40, and 80 ppm on lung (Rl), tissue (Rti), and airway (Raw) resistance in 13 2-5-d-old anesthetized, ventilated, open-chested piglets. Rl was measured from transpulmonary pressure and air flow. Rti was measured by alveolar capsules, and Raw was calculated as the difference between Rl and Rti. Any given concentration of inhaled NO (20, 40, or 80 ppm) significantly decreased Rl (p< 0.001), Rti (p < 0.001), and Raw (p< 0.05). In addition, blockade of endogenous NO with 30 mg/kg Nω-nitro-L-arginine methyl ester (L-NAME) given i.v. in 12 piglets significantly increased Rti and Rl with variable changes in Raw, and caused a decrease in dynamic compliance. Readministration of NO to eight piglets induced a significant decrease in Rl and Rti at 20 and 80 ppm, whereas Raw significantly decreased only at 80 ppm. Pulmonary arterial pressure decreased after exposure to inhaled NO and increased after L-NAME administration. Systemic arterial pressure was unaffected by inhaled NO but increased after L-NAME administration. Our results indicate that Rl, Raw, and Rti are reduced by exogenous NO, suggesting NO-mediated airway smooth muscle relaxation throughout the newborn lung. In contrast, blockade of endogenous NO significantly increases only Rti, suggesting a physiologic role for endogenous NO in regulation of peripheral contractile elements. We speculate that NO-mediated modulation of resistance in pulmonary parenchyma may serve to regulate the balance of ventilation and perfusion and resultant gas exchange in the lungs during early postnatal development.
Similar content being viewed by others
Main
Despite its potential clinical use as a selective pulmonary vasodilator in the treatment of persistent pulmonary hypertension of the newborn, little is known about the changes inhaled NO exerts on Raw and Rti in the newborn lung(1–3). In addition to lowering pulmonary arterial resistance, previous studies report that inhaled NO produces bronchodilatation, reduces histamine- or methacholine-induced bronchoconstriction, and improves ventilation/perfusion matching in adult humans and experimental animals(4–10). There are, however, no reports describing the effect of NO on newborn lung mechanics, and the site of action for any decrease in Rl induced by exogenous NO has yet to be identified.
Endogenous NO is produced in cells by the enzyme NOS, which is found within lung epithelium, airway and vascular smooth muscle, neural tissue, and the endothelium of large pulmonary vessels(11). Endogenous NO may contribute to pulmonary vascular dilatation and airway smooth muscle relaxation and has been also proposed as the mediator for iNANC bronchodilation in adult humans(12–14). Maturational studies suggest that NOS expression and activity are greatest in fetal life and around term gestation, and subsequently decrease with age in endothelial and airway epithelial tissues(15–19). Parenchymal viscoelastic properties and airway caliber are both known to contribute to pulmonary resistance during early postnatal life(20, 21). Viscous resistance is the frictional resistance within lung tissue that is being inflated or deflated. In this study, we used alveolar capsules situated over the lung parenchyma for measuring flow-resistive pressure changes across tissue expressed as lung tissue resistance. This allowed us to partition Rl into its airway and tissue components to characterize the sites of changes in resistance induced by exogenous and endogenous NO. We hypothesized that both exogenous and endogenous NO would modulate baseline pulmonary resistance by affecting airway as well as lung tissue resistance in the newborn piglet.
METHODS
Experimental preparation. Experiments were performed in 13 piglets at 2-4 d of life weighing 2.4 ±.3 kg (mean ± SD). The piglets were initially sedated with intramuscular ketamine hydrochloride (14 mg/kg) and xylazine (2.8 mg/kg) and were anesthetized with i.v.α-chloralose (24 mg/kg) and urethane (120 mg/kg), with additional doses of maintenance anesthesia at 10% of loading dose given approximately every 45 min, depending on acute heart rate and blood pressure changes. A femoral artery was cannulated for measurement of systemic blood pressure and blood gas sampling, and an external jugular vein was cannulated for administration of further anesthesia, NOS blocker, and fluids. A catheter was placed in the pulmonary artery to measure pulmonary arterial pressure.
The piglets were placed on a heating pad to maintain body temperature between 37.5 and 38.5 °C. After a high cervical tracheostomy, the piglets were artificially ventilated with 100% O2 through a tightly fitting tracheal cannula with a side tap connected to a volume ventilator (Harvard model 55-0798) that delivered a tidal volume of 10-12 mL/kg. The ventilator rate was set at approximately 25 cycles/min to maintain an arterial Pco2 of 30-35 mm Hg and an arterial Po2 of >300 mm Hg during baseline conditions. Blood gas tensions and pH were determined with a Radiometer automated blood gas analyzer (ABL3, Copenhagen, Denmark).
Lung mechanics were measured as described previously by ourselves and others(20–23). Briefly, a midline sternotomy was performed, and the chest was widely retracted. Light-weight round-base capsules (10 mm diam) were applied to the pleural diaphragmatic surface of both lungs by means of cyanoacrylate glue. The pleura under each capsule was punctured to a depth of 1-2 mm four to five times with a 20-gauge needle to bring the underlying alveoli into communication with the capsule chamber. Pressures in the capsules and in the tracheal side tap were measured with miniature piezoresistive pressure transducers (model 8510 B-2, Endevco, San Juan Capistrano, CA). Dynamic matching of the transducers was tested, and there was no phase or amplitude distortion of signals in the range of applied pressure and frequency.
We considered that capsules were successfully installed if during slow tidal mechanical ventilation the changes in tracheal and alveolar pressures were eqal at end inspiration and end expiration when there was no flow (Fig. 1). A significant concern of the capsule technique is that pressure within the capsule may not dynamically equal the alveolar pressure beneath the capsule. This discrepancy between alveolar and capsule pressures depends on the time constant of the capsule system, defined by the combined compliance of the transducer and air in the capsule and the resistance to air flow through the pleural openings. For frequencies less than 1 Hz, as used in this study, the capsule pressure should accurately estimate regional alveolar pressure(24). Resistance calculated from the two capsules exhibited minimal differences and was averaged for each experiment. Based on our previous experience, Rti and Raw had comparable results whether derived from an individual capsule or averaged from two capsules(25). The animals were all mechanically ventilated at a positive end-expiratory pressure of 3 cm H2O to prevent closure of the most distal airways. In addition, the lungs were inflated every 10 min by occluding the expiratory line of the ventilator for two to three consecutive volume cycles. Each hyperinflation was performed at an identical time before any experimental maneuver (e.g. NO administration) to avoid confounding effects of different volume histories. The expiratory line was reopened when transpulmonary pressure reached approximately 25 cm H2O.
Tracheal flow signals were obtained with a Fleisch pneumotachograph and were electrically integrated to derive volume. Pressure and flow signals were filtered electrically with matched 15-Hz low pass filters and were recorded on a Gould (Cleveland, OH) six-channel recorder along with pulmonary and systemic arterial blood pressure. The coefficient of variation for 15 control breaths was minimal (0.04 for both Rl and Rti, and 0.006 for Cdyn) during baseline conditions. To rapidly detect possible capsule malfunction, alveolar pressures were displayed against tidal volume on a Tektronix storage oscilloscope. All data were recorded on an FM magnetic tape for later playback and analysis.
A custom-made computer program was used to calculate resistance on the basis of the method of Von Neergaard and Wirz(26). Total Rl and Rti were calculated from tracheal flow and tracheal and alveolar pressure changes, respectively. Raw was derived as the difference between Rl and Rti (Raw = Rl - Rti). Cdyn was calculated as a ratio between volume and pressure measured at tracheal opening. All studies were approved by the Case Western Reserve University Institutional Review Board.
Experimental protocol. In all 13 piglets, baseline measurements of lung mechanics were made over the last minute of a 5-min period of quiet ventilation during inhalation of 100% O2. NO inhalation was used in 12 animals. In 10 piglets, inhaled NO was sequentially administered at 20, 40, and 80 ppm to the inspired gas consisting of 100% O2. In two additional piglets, the three NO dosages were administered in a random sequence. Intervals of 5 min were used between treatments, and measurements of Rl, Raw, Rti, and Cdyn were repeated over 60-s periods after 5 min of exposure to each concentration of NO. The NO dosages used are the same as intially used in the Neonatal Inhaled Nitric Oxide Study conducted by the NICHD Neonatal Research Network. NO concentration was measured by continuously sampling the inspired gas at the airway opening by means of an NO analyzer (Ecophysics; Switzerland), and NO2 concentrations were measured in two piglets using the same analyzer. NO2 concentrations of 0.3-2.5, 8-9, and 10-15 ppm were recorded during delivery of 20, 40, and 80 ppm NO, respectively. After discontinuing NO administration, the piglets were ventilated with 100% O2, and flow and pressure measurements were again recorded to ensure that baseline conditions were restored. The NOS blocker L-NAME at 30 mg/kg was given i.v. to 12 piglets, and Rl, Rti, Raw, and Cdyn were again measured after 5 min.
To determine whether changes induced by blockade of NOS could be reversed by exogenous NO, inhaled NO at 20 and 80 ppm was then readministered to eight piglets, and the same measurements were made. Throughout the experiment, pulmonary and systemic arterial pressures were recorded. Arterial blood gas tensions were checked periodically and did not change significantly during any experiment, with arterial Po2 consistently in the hyperoxic range.
Statistics. Statistical analyses of changes in pulmonary mechanics in response to NO inhalation used one factor repeated measure analysis of variance. Comparisons of measured parameters before and after L-NAME used two-tailed paired t test. Data are expressed as mean± SD. Differences were considered significant at a p < 0.05.
RESULTS
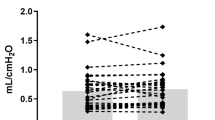
Addition of NO to the inspired gas decreased both the airway and tissue components of Rl. Inhalation of NO at 20, 40, and 80 ppm caused a significant decrease in Rl (p < 0.001), Raw(p < 0.05), and Rti (p < 0.001) in comparison to initial control measurements (Fig. 2). At each concentration of NO, resistance values were significantly lower than during the initial control period. These results did not differ in the subset of animals who received randomized dosages. Increasing NO concentration from 20 to 80 ppm was not followed by further decreases in Rl, Raw, or Rti. Control values before and after termination of NO inhalation also did not differ. Cdyn tended to increase at all dosages, but only at 40 ppm was the increase significant (p < 0.05). Systemic arterial pressure did not change significantly in response to NO inhalation and fluctuated between 60 and 85 mm Hg. Pulmonary arterial pressure, however, demonstrated a significant reduction in response to NO, falling from 17.4± 4.1 to 14.6 ± 3.0 mm Hg after NO was added (p < 0.001). An example of the responses of tracheal and alveolar pressures to NO inhalation is shown in Figure 3.
An example of the responses of tracheal pressure(Ptr), alveolar pressures (Palv), and air flow (˙V) to NO administration at 20 and 80 ppm before and after L-NAME administration. The slight decrease in ˙V as measured by the tracheal pneumotachometer during constant tidal volume ventilation at 80 ppm(but not 20) is probably due to the change in gas density as NO (mixed with N2) is added to the inspired gas.
Blockade of NOS increased both the total and tissue component of Rl. After L-NAME administration, Rti and Rl increased significantly(p = 0.005 and 0.02, respectively), whereas Raw, derived from Rl - Rti, demonstrated a variable response when compared with control values before L-NAME (Table 1). Dynamic lung compliance decreased significantly (p < 0.0001) after L-NAME administration, falling 13% from baseline. L-NAME administration increased both systemic arterial pressure from 66.4 ± 8.1 to 98.9 ± 10.2 mm Hg (p < 0.001) and pulmonary artery pressure from 17.3± 4.4 to 26.3 ± 9.1 mm Hg (p < 0.001).
Readministration of NO at 20 and 80 ppm to eight piglets after L-NAME caused Rl to fall from 21.6 ± 5.8 to 16.5 ± 4.4(p < 0.001) and 14.9 ± 3.5 (p < 0.001) cm H2O/L/s, and Rti to fall from 13.6 ± 5.3 to 11.3 ± 5.5 (p < 0.01) and 9.6 ± 4.4 cm H2O/L/s(p < 0.05) at 20 and 80 ppm, respectively. Raw changed from 9.3 ± 4.3 to 6.8 ± 3.3 cm H2O/L/s (NS) at 20 ppm of NO and significantly fell to 6.2 ± 2.6 cm H2O/L/s (p< 0.05) at 80 ppm of NO. Cdyn was significantly increased from 5.5± 0.9 to 6.2 ± 1.2 mL/cm H2O at 20 ppm (p < 0.01) and 6.3 ± 1.2 mL/cm H2O at 80 ppm (p < 0.001). The responses of tracheal and alveolar pressures to administration of L-NAME followed by inhaled NO in one animal are demonstrated (Fig. 3).
DISCUSSION
Despite the explosion of information concerning the physiologic function and therapeutic uses of NO, to our knowledge there are no published reports dealing with the effects of NO on pulmonary function in the newborn. In this study we have demonstrated that inhaled NO reduces Rl in the newborn by affecting both Raw and Rti. In contrast, inhibition of endogenous NOS caused a significant increase in total Rl by increasing Rti. No consistent effect on Raw was observed.
Previous studies in adults report a bronchodilatory role for inhaled NO. After NO inhalation, Dupuy et al.(5) demonstrated a reduction in Rl in adult guinea pigs whereas Hogman et al.(4) and Sanna et al.(27) reported a small increase in specific airway conductance (reciprocal of resistance) in adult human volunteers with asthma and methacholine-induced bronchospasm. The physiologic effects of inhaled NO on pulmonary resistance in normal adult humans and pediatric asthmatic patients appear to be minimal(27, 28). Based on their finding that NO inhalation did not change resistance in adult rabbits after methacholine nebulization, Hogman et al.(29) suggested that the action of NO was predominantly in the central airways. However, Gwyn et al.(7) recently documented that inhaled NO acted as a bronchodilator of peripheral airways in mature dogs. In our study, we have demonstrated for the first time a decrease in both Raw and Rti using capsules placed on the lungs of open-chested newborn piglets during NO inhalation. Our protocol did not allow us to demonstrate the minimal dose of NO needed to decrease Rl. Whether the mechanisms responsible for bronchodilation are the same as the mechanisms responsible for lowering pulmonary arterial pressure remains to be determined. The presence of NO2 (an upper respiratory tract irritant and bronchoconstrictor) in the inhaled gas may have lessened the magnitude of NO-induced bronchodilation in the lung, and may have attenuated the ability of NO to lower Rl and/or increase Cdyn significantly at high doses of NO(30). Furthermore, low resting bronchomotor tone, partly due to mechanical ventilation and low Paco2, may have limited the ability of NO to induce bronchodilatation(21, 31).
If inhaled NO is able to act as a bronchodilator as well as a vasodilator, this may have important implications for matching ventilation and perfusion throughout the lung. Putensen et al.(8) described improved ventilation/perfusion distribution with NO inhalation in pigs during methacholine-induced bronchoconstriction. A recent report describing improved oxygenation after NO inhalation in newborn piglets with meconium aspiration suggested that selective bronchodilation and the resultant vasodilation in ventilated alveoli improved ventilation/perfusion ratios(32). Further studies are, however, clearly needed to assess the role of exogenous NO in modulating ventilation/perfusion matching under various pathophysiologic conditions.
Our experiment also demonstrates that endogenously produced NO is a determinant of baseline Rl. Because L-NAME infusion increased Rl by increasing Rti, the effect of endogenous NO is physiologically limited to the distal contractile elements of the newborn piglet and actively reduces baseline resistance at this level but not at the larger airways. We speculate that NOS blockade, by decreasing endogenous NO production in the newborn lung, increases Rti by promoting constriction of distal smooth muscle and/or interstitial contractile elements. The fall in dynamic lung compliance after L-NAME infusion is consistent with previous data that demonstrate a simultaneous increase in Rti(20, 25).
Using adult subjects, several studies investigating the role of endogenous NO on pulmonary function have demonstrated conflicting findings. In contrast to our results, Kips et al.(33) infused L-NAME into adult rats and demonstrated no effect on basal airway tone or basal airway responsiveness. It must be noted, however, that the varying forms of NOS are expressed within the lung at different levels throughout maturation. Levels of NOS have been shown to increase during gestation and subsequently decrease with age(15–19). Our results suggest that the significant increase in Rti after NOS blockade is due to differences in expression of NOS in the newbron compared with the adult. The absence of a significant effect of endogenously released NO on baseline Raw may be explained by a low airway smooth muscle tone in the newborn piglet(34). In this study we did not have an independent assessment of the contractile state of the airways under control conditions. The modest increase in Rti observed after NOS blockade is probably not of great physiologic significance, however, under pathologic conditions associated with increased airway tone, an opposing airway relaxation induced by release of endogenous NO may assume much greater prominence. Recently we have shown that NOS blockade significantly potentiates airway smooth muscle responses to bronchoconstrictive agents in early postnatal life(35). The ability of inhaled NO at 20 and 80 ppm to reverse the effects of NOS blockade on Rti further supports a role for endogenous NO in modulating baseline pulmonary function.
Endogenous NO production and its contribution to iNANC bronchodilation have been demonstrated in adult subjects, but its presence and activity in newborns has been uncertain. Studies in rabbits suggest that the iNANC system in rabbits is not functional at birth and undergoes considerable postnatal development, whereas the iNANC system does exist in pigs and newborn cats, suggesting significant interspecies differences(36–38). Our findings that NOS inhibition produced an elevation of Rti in newborn pigs suggest that baseline iNANC activity is measurable in more distal airway and lung parenchyma in that species. Recently we demonstrated that piglet lung parenchymal tissue is modulated by central cholinergic output. Hence an opposing relaxant system may counteract constrictor influences at this location(21). In adults, Ward et al.(39) showed that NO-mediated iNANC bronchodilation is present throughout the tracheobronchial tree, but with decreased response in the distal airways secondary to decreased NOS-containing nerves. Future experiments will need to correlate location of physiologic responses with localization of NOS-containing structures throughout the developing lung.
The use of alveolar capsules allowed the partitioning of Rl into tissue and airway components. We believe that the current study accurately demonstrated airway and lung partitioning, because Rti and Raw contributed more equally to Rl under baseline conditions as we previously described for the newborn piglet(25). The actual changes observed at the tissue level may be due to diverse distal contractile elements, including bronchiolar smooth muscle, interstitial cells, and the distal pulmonary vasculature(21, 22). We cannot identify which contractile elements are responsible for the change induced by NO and changes in the state of pulmonary vascular constriction could possibly account for the changes in tissue viscance induced by NO or NOS blockade. As L-NAME increased Rti without a significant increase in Raw, tissue distortion secondary to changes in resistance of larger airways probably cannot explain our findings. It is possible that ventilation at relatively high tidal volumes may have limited bronchoconstriction induced by L-NAME, by inhibiting airway smooth muscle contraction as has been described in immature rabbits when alveolar capsules were used(22). High concentrations of bronchoconstrictive agents may induce the development of inhomogenous ventilation, leading to inaccurate assessment of lung mechanics by alveolar capsules(23). By maintaining constant ventilator settings and observing identical responses from two capsules placed on different lung lobes, we have minimized the likelihood of such inhomogeneity. Furthermore, the mild constrictive effects induced by L-NAME would be unlikely to induce detectable inhomogeneity. Throughout our experiment, responses of systemic and pulmonary artery pressures to NO were consistent with previous findings and arterial blood gas tensions did not physiologically vary significantly during the experiment. Inhaled NO produced a selective decrease in pulmonary artery pressure, whereas L-NAME increased both pulmonary and systemic artery pressure(1, 2, 40, 41). We therefore cannot exclude NO mediated pulmonary arteriolar and venular vasodilation with a resultant increase in pulmonary blood flow (or vasoconstriction after NOS blockade) as possible contributors to our results. There is the additional possibility that NO-mediated mechanisms may alter the properties of surface-active molecules, resulting in changes in Rti(42). Future in vitro studies using distal airway (or vascular) segments or lung parenchymal strips might enable us to identify whether airway smooth muscle contraction is the primary mechanism underlying the observed responses of Rti.
Our study demonstrates a contribution of NO to airway smooth muscle relaxation and change in tissue viscance in the newborn lung with resultant implications for pulmonary function. Inhaled NO in the newborn piglet caused a significant decrease in resistance throughout the lung. Blockade of endogenous NO produced a significant increase in only Rti, implicating a relaxant effect of endogenous NO on lung parenchyma rather than larger airways under baseline conditions. Greater understanding of the location of relaxant responses induced by No may clarify our understanding of ventilation/perfusion balance in both health and disease.
Abbreviations
- NO:
-
nitric oxide
- NOS:
-
nitric oxide synthase
- NO2:
-
nitrogen dioxide
- Rl:
-
lung resistance
- Raw:
-
airway resistance
- Rti:
-
tissue resistance
- iNANC:
-
inhibitory nonadrenergic noncholingeric
- Cdyn:
-
dynamic compliance
- L-NAME:
-
Nω-nitro-L-arginine-methyl ester
References
Roberts JD, Polaner DM, Lang P, Zapol WM 1992 Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340: 818–819.
Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM 1991 Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83: 2038–2047.
Kinsella JP, Abman SH 1995 Recent developments in the pathophysiology and treatment of persistent pulmonary hypertension of the newborn. J Pediatr 126: 853–864.
Hogman M, Frostelll C, Hedenstrom H, Hedenstierna G 1993 Inhalation of nitric oxide modulates adult human bronchial tone. Am Rev Respir Dis 148: 1474–1478.
Dupuy PM, Shore SA, Drazen JM, Frostell C, Hill W, Zapol WM 1992 Bronchodilator action of inhaled nitric oxide in guinea pigs. J Clin Invest 90: 421–428.
Brown RH, Zerhouni EA, Hirshman CA 1994 Reversal of bronchoconstriction by inhaled nitric oxide: Histamine versus methacholine. Am J Respir Crit Care Med 150: 233–237.
Gwyn DR, Lindeman KS, Hirshman CA 1996 Inhaled nitric oxide attenuates broncho-constriction in canine peripheral airways. Am J Respir Crit Care Med 151: 604–609.
Putensen C, Räsänen J, López FA 1995 Improvement in VA/Q distributions during inhalation of nitric oxide in pigs with methacholine-induced bronchoconstriction. Am J Respir Crit Care Med 151: 116–122.
Pison U, Lopez FA, Heidelmeyer CF, Rossaint R, Falke KJ 1993 Inhaled nitric oxide reverses hypoxic pulmonary vasoconstriction without impairing gas exchange. J Appl Physiol 74: 1287–1292.
Abman SH, Griebel JL, Parker DK, Schmidt JM, Swanton D, Kinsella JP 1994 Acute effects of inhaled nitric oxide in children with severe hypoxemic respiratory failure. J Pediatr 124: 881–888.
Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS 1993 Nitric Oxide Synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol 9: 371–377.
Moncada S, Higgs A 1993 The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012.
Barnes PJ 1993 Nitric oxide and airways. Eur Respir J 6: 163–165.
Belvisi MG, Stretton CD, Yacoub M, Barnes PJ 1992 Nitric oxide is the endogenous neurotransmitter of bronchodilator nerves in humans. Eur J Pharmacol 210: 221–222.
Kawai N, Bloch DB, Filippov G, Rabkina D, Suen H, Losty PD, Janssens SP, Zapol WM, DeLaMonte S, Bloch KD 1995 Constitutive endothelial nitric oxide synthase gene expression is regulated during lung development. Am J Physiol 268:L589–L595.
North AJ, Star RA, Brannon TS, Ujiie K, Wells LB, Lowenstein CJ, Snyder SH, Shaul PW 1994 Nitric oxide synthase type I and type III gene expression are developmentally regulated in rat lung. Am J Physiol 266:L635–L641.
Xue C, Reynolds PR, Johns RA 1996 Developmental expression of NOS isoforms in fetal rat lung: implications for transitional circulation and pulmonary angiogenesis. Am J Physiol 270: L88–L100.
Halbower AC, Tuder RM, Franklin WA, Pollock JS, Förstermann U, Abman SH 1994 Maturation-related changes in endothelial nitric oxide synthase immunolocalization in developing ovine lung. Am J Physiol 267:L585–L591.
Buttery LDK, Springall DR, daCosta FAM, O Oliveira H, Hislop AA, Haworth SG, Polak JM 1995 Early abundance of nerves containing NO synthase in the airways of newborn pigs and subsequent decrease with age. Neurosci Lett 201: 219–222.
Dreshaj IA, Martin RJ, Miller MJ, Haxhiu MA 1994 Responses of lung parenchyma and airways to tachykinin peptides in piglets. J Appl Physiol 77: 147–151.
Martin RJ, Dreshaj IA, Miller MJ, Haxhiu MA 1995 Neurochemical control of tissue resistance in piglets. J Appl Physiol 79: 812–817.
Tepper RS, Shen X, Bakan E, Gunst SJ 1995 Maximal airway response in mature and immature rabbits during tidal ventilation. J Appl Physiol 79: 1190–1198.
Fredberg JJ, Ingram RH Jr, Castile RG, Glass GM, Drazen JM 1985 Nonhomogeneity of lung response to inhaled histamine assessed with alveolar capsules. J Appl Physiol 58: 1914–1922.
Fredberg JJ, Keefe DH, Glass GM, Castile RG, Frantz ID 1984 Alveolar pressure nonhomogeneity during small-amplitude high-frequency oscillation. J Appl Physiol 57: 788–800.
Dreshaj IA, Haxhiu MA, Potter CF, Agani FH, Martin RJ 1996 Maturational changes in responses of tissue and airway resistance to histamine. J Appl Physiol 81: 1785–1791.
Von Neergaard K, Wirz K 1927 Uber eine Methode zur Messung der Lungenelastizitat am lebenden Menschen, insbesondere beim Emphysem. Z Klin Med 105: 35–50.
Sanna A, Kurtansky A, Veriter C, Stanescu D 1994 Bronchodilator effect of inhaled nitric oxide in healthy men. Am J Respir Crit Care Med 150: 1702–1704.
Pfeffer KD, Ellison G, Robertson D, Day RW 1996 The effect of inhaled nitric oxide in pediatric asthma. Am J Respir Crit Care Med 153: 747–751.
Hogman M, Frostell C, Arnberg H, Hedenstierna G 1993 Inhalation of nitric oxide modulates methacholine induced bronchoconstriction in the rabbit. Eur Respir J 6: 177–180.
Ellenhorn MJ, Barceloux DG 1988 Medical Toxicology. Elsevier Science Publishing, New York, pp 876–877.
Waldron MA, Fisher JT 1988 Differential effects of CO2 and hypoxia on bronchomotor tone in the newborn dog. Respir Physiol 72: 271–282.
Barrington KJ, Finer NN, Peliowski A, Etches PC, Graham AJ, Chan W 1995 Inhaled nitric oxide improves oxygenation in piglets with meconium aspiration. Pediatr Pulmonol 20: 27–33.
Kips JC, Lefebvre RA, Peleman RA, Joos GF, Pauwels RA 1995 Effect of a nitric oxide synthase inhibitor on the modulation of airway responsiveness in rats. Am J Respir Crit Care Med 151: 1165–1169.
Haxhiu-Poskurica B, Carlo WA, Miller MJ, DiFiore JM, Haxhiu MA, Martin RJ 1991 Maturation of reflex responses in the piglet. J Appl Physiol 70: 608–616.
Jakupaj M, Martin RJ, Dreshaj IA, Potter CF, Haxhiu MA 1996 Nitric Oxide (NO) modulates contractile responses of airway smooth muscle during early postnatal life. Pediatr Res 39: 335A
Colasurdo GN, Loader JE, Graves JP, Larsen GL 1994 Maturation of nonadrenergic noncholinergic inhibitory system in normal and allergen-sensitized rabbits. Am J Physiol 267: L739–L744.
Waldron MA, Connelly BJ, Fisher JT 1989 Nonadrenergic inhibitory innervation to the airways of the newborn cat. J Appl Physiol 66: 1995–2000.
Kannan MS, Johnson DE 1992 Nitric oxide mediates the neural nonadrenergic, noncholinergic relaxation of pig tracheal smooth muscle. Am J Physiol 262: L511–L514.
Ward JK, Barnes PJ, Springall DR, Abelli L, Tadjkarimi S, Yacoub MH, Polak JM, Belvisi MG 1995 Distribution of human i-NANC bronchodilator and nitric oxide-immunoreactive nerves. Am J Respir Cell Mol Biol 13: 175–184.
Rees DD, Palmer RMJ, Shulz R, Hodson HF, Moncada S 1990 Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 101: 746–752.
Jorens PG, Vermeire PA, Herman AG 1993 L-Arginine dependent nitric oxide synthase: a new metabolic pathway in the lung and airways. Eur Respir J 6: 258–266.
Fredberg JJ, Stamenovic D 1989 On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408–2419.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grants HL56470 and HL50527.
Rights and permissions
About this article
Cite this article
Potter, C., Dreshaj, I., Haxhiu, M. et al. Effect of Exogenous and Endogenous Nitric Oxide on the Airway and Tissue Components of Lung Resistance in the Newborn Piglet. Pediatr Res 41, 886–891 (1997). https://doi.org/10.1203/00006450-199706000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199706000-00014
This article is cited by
-
A Randomized, Multicenter, Blinded Pilot Study Assessing the Effects of Gaseous Nitric Oxide in an Ex Vivo System of Human Lungs
Pulmonary Therapy (2023)
-
The effect of inhaled nitric oxide on pulmonary function in preterm infants
Journal of Perinatology (2007)
-
Acute Effects of Inhaled Nitric Oxide on Pulmonary and Cardiac Function in Preterm Infants with Evolving Bronchopulmonary Dysplasia
Journal of Perinatology (2004)