Abstract
The production of IL-10 by newborns has not been studied in much detail. We analyzed the IL-10 production by, and surface marker distribution of, cord blood mononuclear cells (n = 47); adult peripheral blood mononuclear cells (n = 30) were used as controls. Both the baseline (0.79versus 1.54 ng/mL, p = 0.001) and lipopolysaccharide-stimulated (1.20 versus 2.88 ng/mL, p = 0.003) levels of IL-10 production were significantly lower in newborns than in adults. No significant differences were observed after stimulation with concanavalin A. Both cord blood and adult CD19+CD5+ cells were able to produce IL-10; however, the level of production was only an average of 16% of the total stimulated IL-10 production by unfractionated cells, indicating that CD5+ B cells are not the primary source of IL-10 in either adults or newborns. In newborns, the proportion of naive CD4+CD45RA+ cells was inversely correlated with IL-10 response to lipopolysaccharide (r = -0.49, p = 0.004) indicating a role for maturing T cells in neonatal IL-10 production; the number of macrophages was not significantly correlated with IL-10 response (r= 0.30, p = 0.10). In contrast, in adults IL-10 production correlated with the number of macrophages (r = 0.49, p = 0.01) but not CD4+CD45RA+ cells (r = -0.06,p = 0.77). We conclude that newborns produce less IL-10 than adults; the primary cells of origin and the regulatory mechanisms may be different from those observed in adults.
Similar content being viewed by others
Main
IL-10 is a cytokine that was initially found to inhibit cytokine secretion by Th1 type T cells(1) through specific inhibition of IL-2 production(2). It soon became clear that IL-10 is a pleiotropic cytokine produced not only by Th2 cells but also by many other cells(3–5).
The production of IL-10 by newborns has not been studied in much detail. One previous report indicates that a proportion of neonates produce high amounts of IL-10(6). In mice, the majority of B cell-derived IL-10 is produced by CD5+ B cells (B-1 cells) and IL-10 is considered an autocrine growth factor to these cells(7). Human newborns have significantly more CD5+ B cells than adults(8), which might explain the observed increased IL-10 production. Increased IL-10 production could, in turn, contribute to the impaired production of some other cytokines, such as IFN-γ, observed in newborns(9, 10). However, this model is not in concordance with the finding that cord blood mononuclear cells do produce adequate amounts of IL-2 after mitogen stimulation(11), indicating an absence of any inhibitory effects of IL-10. Indeed, it would also be reasonable to expect not increased, but rather defective IL-10 production by cord blood cells not only because the production of many other cytokines is decreased in newborns, but also because the majority of cord blood T cells are CD45RA+(8), a cell type shown in adults to be defective for IL-10 production(3). To elucidate this controversy, we have examined IL-10 production by cord blood and adult mononuclear cells.
METHODS
Cord blood samples from 47 healthy, full-term newborns were collected from umbilical cord vessels within minutes after normal delivery (nine cases) or elective cesarean section (38 cases). The study protocol was approved by the ethical committees of the Children's Hospital, and of the Department of Obstetrics and Gynecology, University of Helsinki. Peripheral blood samples obtained from 30 healthy adults were used as controls.
Mononuclear cells were isolated from peripheral or cord blood by a Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient. The cells (1× 106/mL) were cultured at 37°C in RPMI 1640 (Life Technologies, Inc., Paisley, Scotland) + 10% FCS + 1% glutamine + 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate (all from Life Technologies), for 72 h, the optimal time point based on both earlier observations(6) and our own results demonstrating peak IL-10 supernatant concentrations at 48-72 h with no statistically significant difference between these two time points. The cells were stimulated at 0 h by adding either LPS (final concentration, 5 μg/mL) (Sigma Chemical Co., St. Louis, MO), ConA (5 μg/mL) (Sigma Chemical Co.), or, in some experiments, polyclonal anti-IgM antibodies (10 μg/mL) (Protos ImmunoResearch, San Francisco, CA).
CD5+ B cell isolation (three neonates and two adults) was performed in two steps by first incubating mononuclear cells with magnetizable polystyrene beads coated with anti-CD19 MAb (Dynabeads® M-450 PanB (CD19), Dynal, Oslo, Norway) for 20 min at 4°C. Cells attached to the magnetic beads were captured with a magnetic particle concentrator MPC©-6 (Dynal). Magnetic beads were removed by applying polyclonal antibody specially made for detachment of these beads (DETACHaBEAD®, Dynal). The proportion of CD19+ cells of all recovered cells, as analyzed by flow cytometry, was 85-98%. For further isolation of CD5+ B cells, magnetic beads coated with rat anti-mouse IgG1 were first incubated with mouse-anti-human-CD5+ antibodies (DAKO, Glostrup, Denmark) and then with CD19+ cells as advised by the manufacturer (Direct technique, Dynal). Cells attached to the beads were captured with MPC-6.
Concentration of IL-10 in the culture supernatants was determined by an ELISA. Briefly, microtiter plates (Nunc-Immuno Plate, Roskilde, Denmark) were coated overnight at 4°C with rat anti-human IL-10 (10 μg/mL)(Pharmingen, San Diego, CA). Samples (1/1 and 1/5) and standards (Pharmingen) were diluted in RPMI 1640 + 5% FCS. Biotinylated rat anti-human IL-10 (2μg/mL) (Pharmingen) and streptavidin-HRP (Zymed, South San Francisco, CA) were used for detection of IL-10. The detection limit was 0.35 ng/mL.
For surface marker analysis, 50 μL of whole blood were incubated with 10μL of MAb specific for CD3, CD4, CD8, CD5, CD19, CD14, CD45RA, CD45RO, or CD16+CD56 (Immunotech, Marseille, France). Red cells were lysed with fluorescence-activated cell sorter lysing solution (Becton Dickinson, San Jose, CA), and the cells were fixed according to the manufacturer's instructions. Cells were analyzed with a FAC-Scan flow cytometer (Becton Dickinson).
The association between IL-10 production in response to LPS or ConA and proportion of T and B cells, natural killer cells, and macrophages, was assessed by linear correlation analysis (a logarithmic transformation of the response was used to normalize the distribution). To compare the results between adults and newborns, a Mann-Whitney U test (IL-10 production) and t test (surface markers) were used. The resulting two-tailed p values are unadjusted for multiple comparisons.
RESULTS
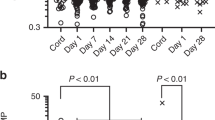
Both the baseline (Fig. 1) and LPS-stimulated levels of IL-10 production were significantly lower in newborns than in adults. However, there was no significant difference in the relative magnitude of the response (Table 1). When cord blood cells were stimulated with ConA, neither the magnitude of the response nor the actual level of IL-10 production were statistically significantly different from those of the adults.
As expected, the neonates had more B cells, especially CD5+ B cells, and significantly less CD45RO+ T cells than did adults(Table 2). Significant differences were also found in the proportions of macrophages and CD3+ cells. In adult controls, the proportions of both macrophages (r = 0.49, p = 0.01) and B cells (r = 0.71, p < 0.001) were correlated with the IL-10 response to LPS, indicating that these cells are a significant source of IL-10. When analyzed in more detail, only the proportion of CD5- B cells, but not the proportion of CD5+ B cells, was correlated with the IL-10 response (r = 0.72, p < 0.001, and r =-0.15, p = 0.46, respectively). No correlations between other surface markers and IL-10 response to LPS were observed in adults.
In newborns, there were no significant correlations between IL-10 response to LPS and the proportion of either macrophages (r = 0.30,p = 0.10) or B cells (r = -0.03, p = 0.90). However, there was an inverse correlation between IL-10 response and the proportion of CD5+ B cells (r = -0.44, p = 0.01) and a positive correlation between IL-10 response and the proportion of CD5- B cells (r = 0.44, p = 0.01). Further, the proportion of CD4+CD45RA+ cells was inversely correlated with IL-10 response in newborns (r = -0.49, p = 0.004) but not in adults (r = -0.06, p = 0.77). No significant correlations between surface marker distribution and IL-10 production in response to ConA were observed in either newborns or in adults.
To examine the relative importance of CD5+ B cells in IL-10 production, we separated CD19+CD5+ cells from three cord blood and two adult samples. The separation process itself resulted in an activation of the baseline IL-10 production in four out of the five individual experiments: the unstimulated adult CD5+ B cells produced 53 and 210% as much IL-10 as unstimulated unfractionated mononuclear cells, respectively, whereas the cord blood CD5+ B cells produced 110, 380, and 515% as much IL-10 as unfractionated cells, respectively. These results indicate that, even if no further activation in response to either LPS or anti-IgM antibodies occurred in any of the five experiments, CD5+ B cells can produce IL-10 in both newborns and adults. On the other hand, the magnitude of this production was only an average of 16% (in adults, 11 and 12%, and in newborns, 4, 20, and 33%, respectively) of the total stimulated IL-10 production by unfractionated cells, indicating that CD5+ B cells are not the primary source of IL-10 in either adults or newborns.
DISCUSSION
We have demonstrated that, on the average, cord blood mononuclear cells produce less IL-10 than adult peripheral blood mononuclear cells. However, there were many newborns who produced more IL-10 than many adults did. It is known that, during severe infections, serum IL-10 levels are elevated(12–14). Consequently, the observed high levels of neonatal IL-10 production in both one previous(6) and the present study might result from an infection of either the newborn or the cord blood sample; on the other hand, in our study all neonates were healthy, and all blood samples were processed within 4 h.
Interestingly, the relative magnitude of the IL-10 response (expressed as percent of baseline production) to both LPS and ConA in newborns was not significantly different from that in adults. This could be interpreted as indicating that the qualitative differences between newborns and adults in the network regulating IL-10 production may not be significant. Rather, the decreased ability to produce IL-10 might be due to a global immaturity of cord blood cells leading to a decreased ability to produce any cytokines, with the possible exception of IL-2(11). The global immaturity would also explain why-despite IL-10 and IFN-γ down-regulating each other in adults-both IL-10 and IFN-γ production are decreased in newborns.
We were not able to exclude the possibility that, in fact, IL-10 production in newborns is normal or even supranormal, but increased consumption by,e.g. CD5+ B cells results in decreased supernatant IL-10 concentrations. The problem of demonstrating decreased production-as opposed to increased consumption-could be approached by using a semiquantitative reverse transcriptase-polymerase chain reaction. However, even using the reverse transcriptase-polymerase chain reaction would not solve the problem completely: it is entirely possible that, despite normal or even supranormal mRNA levels, translation of IL-10, or any other protein, is impaired in newborns with resulting decreased levels of produced IL-10. In future studies, intracellular staining methods(15, 16) will allow for demonstration of translated protein, but even then, defective secretion cannot be ruled out. These difficulties are not unique to the present report, but hamper all studies on cytokine production.
Although in mice the CD5+ B cells produce the majority of B cell-derived IL-10, these cells did not appear to be important sources of IL-10 in either newborns, as indicated by the inverse correlation between the number of CD5+ B cells and IL-10 production, or in adults. Data obtained from experiments using isolated CD5+ B cells confirm this impression: although both cord blood and adult CD5+ B cells were able to produce IL-10, the level of production was nonetheless significantly lower than that produced by unfractionated cells. However, there are certain caveats one needs to keep in mind. CD5+ B cells might need some external help from other cells for satisfactory IL-10 production, rendering experiments with isolated cells less important. In addition, autoconsumption of IL-10 may mask increased production. On the other hand, it is possible that the CD19+CD5+ cell fraction contained some macrophages that, in fact, were at least partially responsible for the observed IL-10 production.
In adults, T cells produce significant amounts of IL-10; however, the naive CD4+CD45RA+ cells have an impaired ability to produce IL-10(3). A role for maturing T cells in neonatal IL-10 production is indicated by the association between the proportion of naive T cells and the IL-10 response to LPS: the smaller the proportion of naive T cells in cord blood, the better IL-10 production. Of course, the type of stimulation may have a crucial effect in determining which cell type(s) will be activated; as an example, it has recently been reported that neonates do not respond to LPS in the absence of CD14+ cells(17). Unfortunately, due to the small number of neonatal cells available, we were unable to perform more thorough studies comparing different types of stimulation.
We conclude that cord blood cells produce less IL-10 than adult mononuclear cells. This might be due to a global immaturity of cord blood cells leading to a decreased ability to produce any cytokines. The cells of origin of neonatal IL-10 remain to be determined; based on the available data, CD5+ B cells are not the primary source.
Abbreviations
- LPS:
-
lipopolysaccharide
- ConA:
-
concanavalin A
- IFN:
-
interferon
References
Fiorentino DF, Bond MW, Mosmann TR 1989 Two types of mouse T helper cell. J Exp Med 170: 2081–2095.
de Waal Malefyt R, Yssel H, de Vries JE 1993 Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. J Immunol 150: 4754–4765.
Yssel H, de Waal Malefyt R, Roncarolo M-G, Abrams JS, Lahesmaa R, Spits H, de Vries JE 1992 IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol 149: 2378–2384.
Mosmann TR 1994 Properties and functions of interleukin-10. Adv Immunol 56: 1–26.
Rivas MR, Ullrich SE 1992 Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. J Immunol 149: 3865–3871.
Abrams JS, Roncarolo M-G, Yssel H, Andersson U, Gleich GJ, Silver JE 1992 Strategies of anti-cytokine monoclonal antibody development: immunoassays of IL-10 and IL-5 in clinical samples. Immunol Rev 127: 5–24.
O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M 1992 Ly-1 (B-1) cells are the main source of B-cell-derived IL-10. Eur J Immunol 22: 711–717.
Erkeller-Yuksel FM, Deneys V, Yuksel B, Hannet I, Hulstaert F, Hamilton C, Mackinnon H, Stokes LT, Munhyeshuli V, Vanlangendonck F, De Bruyère M, Bach BA, Lydyard PM 1992 Age-related changes in human blood lymphocyte subpopulations. J Pediatr 120: 216–222.
Wilson CB, Westall J, Johnston L, Lewis DB, Dower ST, Alpert AR 1986 Decreased production of interferon-γ by human neonatal cells. J Clin Invest 77: 860–867.
Pirenne-Ansart H, Paillard H, De Groote D, Eljaafari A, Le Gac S, Blot P, Franchimont P, Vaquero C, Sterkers G 1995 Defective cytokine expression but adult-type T-cell receptor, CD8, and p56lck modulation in CD3- or CD2-activated T cells from neonates. Pediatr Res 37: 64–69.
Andersson U, Andersson J, Lindfors A, Wagner K, Möller G, Heusser CH 1990 Simultaneous production of interleukin 2, interleukin 4 and interferon-γ by activated human blood lymphocytes. Eur J Immunol 20: 1591–1596.
Lehmann AK, Halstensen A, Sønes S, Røke O, Waage A 1995 High levels of interleukin 10 are associated with fatality in meningococcal disease. Infect Immunol 63: 2109–2112.
Marchant A, Deviére J, Byl B, De Groote D, Vincent J-L, Goldman M 1994 Interleukin-10 production during septicaemia. Lancet 343: 707–708.
Derkx B, Marchant A, Goldman M, Bijlmer R, van Deventer S 1995 High levels of interleukin-10 during the initial phase of fulminant meningococcal septic shock. J Infect Dis 171: 229–232.
Sander B, Andersson J, Andersson U 1991 Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev 119: 65–93.
Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra A 1995 Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med 182: 1357–1367.
Cohen L, Haziot A, Ren Shen D, Lin X, Sia C, Harper R, Silver J, Goyert SM 1995 CD14-independent responses to LPS require a serum factor that is absent from neonates. J Immunol 155: 5337–5342.
Acknowledgements
The authors express their gratitude to Dr. Sture Andersson at the Department of Obstetrics and Gynecology of the Helsinki University Hospital for helping in collecting the cord blood samples. We also thank an anonymous reviewer for helpful suggestions.
Author information
Authors and Affiliations
Additional information
Supported in part by the Foundation for Pediatric Research and the Clinical Research Institute, Helsinki University Central Hospital.
Rights and permissions
About this article
Cite this article
Kotiranta-Ainamo, A., Rautonen, J. & Rautonen, N. Interleukin-10 Production by Cord Blood Mononuclear Cells. Pediatr Res 41, 110–113 (1997). https://doi.org/10.1203/00006450-199701000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199701000-00017
This article is cited by
-
A dynamic model of gene expression in monocytes reveals differences in immediate/early response genes between adult and neonatal cells
Journal of Inflammation (2007)
-
TNF-α is a mediator of the anti-inflammatory response in a human neonatal model of the non-septic shock syndrome
Pediatric Surgery International (2006)