Abstract
To determine the effect of parenteral nutrition on the balance and catabolism of leucine (by oxidation) and phenylalanine (by hydroxylation) and to assess any acute changes in proteolysis and/or protein synthesis, leucine and phenylalanine kinetics were measured by stable isotope tracer infusions in nine 32-wk gestation premature infants under both basal conditions and in response to an i.v. infusion of glucose, lipid, and amino acids. Leucine and phenylalanine balance both changed from negative to positive during parenteral nutrition. However, leucine and phenylalanine catabolism were differently affected by parenteral nutrition; the rate of leucine oxidation increased 2-fold, whereas the rate of phenylalanine hydroxylation was unchanged from basal values. Phenylalanine utilization for protein synthesis and leucine utilization for protein synthesis (based on both plasma leucine andα-ketoisocaproic acid enrichments) increased significantly during parenteral nutrition. The endogenous rates of release of leucine (based on plasma leucine enrichment) and phenylalanine (both reflecting proteolysis) were significantly reduced during parenteral nutrition. The endogenous rate of release of leucine (based on α-ketoisocaproic acid enrichment) was slightly but not significantly lower during parenteral nutrition. The substantial increase in leucine oxidation without changes in phenylalanine hydroxylation suggests a possible limitation in the phenylalanine/tyrosine supply during parenteral nutrition. In addition, these results suggest that premature infants respond to parenteral nutrition with acute increases in whole body protein synthesis as well as a probable reduction in proteolysis.
Similar content being viewed by others
Main
Intravenous infusions of glucose, lipid, and amino acids are commonly used to provide nutritional support to premature infants. When delivered in adequate quantity, these i.v. infusions produce acceptable rates of growth and protein accretion(1, 2). However, it is uncertain whether current parenteral nutrition solutions provide optimal quantities of specific essential amino acids to replace irreversible losses and support growth. Determining the rates of catabolism of leucine (by oxidation) and phenylalanine (by hydroxylation) in the basal state provides an estimate of minimal requirements for these essential amino acids in premature infants to replace irreversible losses. Furthermore, assessing the changes in the irreversible loss of leucine and phenylalanine in response to parenteral nutrition may provide an indication of whether leucine and phenylalanine are being provided in excessive or possibly limiting amounts for overall protein accretion(3). In concept, excessively provided essential amino acids may exhibit a substantial increase in catabolism, whereas those provided in adequate or limiting amounts may not(3). Therefore, the present study was designed to assess the pattern of leucine and phenylalanine catabolism in premature infants by measuring leucine oxidation and phenylalanine hydroxylation under basal conditions and in response to parenteral nutrition delivered in quantities sufficient to support growth.
In addition to measuring catabolism, assessing whole body proteolysis and protein synthesis using these essential amino acids is also of interest. We have recently demonstrated that parenteral nutrition produces similar increases in protein synthesis in extremely premature (26-wk gestation) and term newborns, but that proteolysis is reduced much less in extremely premature compared with term newborns(4). We speculated that the apparent resistance to suppression of proteolysis may be related to the degree of immaturity. Therefore, a secondary objective of the present study was to assess whether premature infants of intermediate gestational age(≈32 wk) would respond to parenteral nutrition with an intermediate suppression of proteolysis.
METHODS
Subjects. Studies were approved by the Institutional Review Board of Indiana University, and written informed consent was obtained from the parents. Healthy premature newborns between 30 and 34 wk of gestation and less than 14 d of age were eligible for study. The clinical characteristics of the nine study subjects are summarized in Table 1. All infants were appropriate for gestational age, had no congenital anomalies or perinatal asphyxia, and had no clinical evidence of sepsis. Infants were studied at ≤12 d of age, at which time none required the administration of oxygen or ventilator assistance. The premature newborns were provided enteral feedings for 3.6 ± 0.9 d (range 1-9) before study, and received 67± 17 kcal/kg/day (range 9-120) of enteral caloric intake for the 24 h before study. There was no significant relationship between the enteral caloric intake and any of the subsequently measured parameters.
Protocol. Studies were begun 3-4 h after a regular feeding. In infants without i.v. access in place for clinical purposes, an indwelling peripheral catheter was placed in a superficial vein for the infusion of isotopes and parenteral nutrition. A second catheter was placed in the opposite extremity for the purpose of drawing blood. A low infusion rate of glucose was begun and continued throughout the basal period to maintain constant glucose concentrations and prevent hypoglycemia. Fifteen to 30 min after the glucose infusion was begun, a baseline blood sample was obtained. A priming dose of [1-13C]leucine (10 μmol/kg),[d5]phenylalanine (4 μmol/kg),[d2]tyrosine (2 μmol/kg), and[d4]tyrosine (0.5 μmol/kg) dissolved in normal saline was then administered over 5 min. Thereafter, constant infusions of[1-13C]leucine (7 μmol/kg/h), [d5]phenylalanine(2.5 μmol/kg/h), and [d2]tyrosine (1.4 μmol/kg/h) dissolved in normal saline were delivered through the first venous catheter via an infusion pump (Harvard Apparatus, Inc., South Natick, MA) and continued throughout the study period. During the basal period, blood samples were obtained at 140, 160, and 180 min after beginning the isotope infusion and were immediately analyzed for plasma glucose concentration. The remainder of the sample was then frozen at -70 °C for later analysis.
After the 180-min sample, an i.v. solution of glucose (8 mg/kg/min), lipid(165 mg/kg/h)(Liposyn III 10%, Abbott Laboratories, North Chicago, IL), and amino acids (105 mg/kg/h) (Aminosyn-PF, Abbott Laboratories, North Chicago, IL) was begun and continued for the remaining 2.5 h of the study. The amino acid solution provided 95 μmol/kg/h leucine, 27 μmol/kg/h phenylalanine, and 4 μmol/kg/h tyrosine. If this infusion were to continue over a 24-h period, it would provide 90 kcal/kg, 2.5 g/kg of protein, 4 g/kg lipid, and 11.5 g/kg glucose. Blood samples were then obtained at 290, 310, and 330 min, analyzed for plasma glucose, and frozen for later analysis. Syringes containing infusates were weighed before and after infusion to quantitate volume actually delivered, and each infusate was analyzed for the concentration of leucine, phenylalanine, and tyrosine.
Resting oxygen consumption and carbon dioxide production were measured using open circuit respiratory calorimetry as previously described(5). The system was calibrated by combustion of absolute ethyl alcohol, and the results were within 5% of expected values. Respiratory calorimetry was performed throughout the study, and values obtained during the last hour of each study period were used for analysis. Subjects were quiet or sleeping during all measurements.
Analytical methods. Plasma enrichments. The plasma enrichments of leucine, phenylalanine, tyrosine, and KIC were determined by electron impact ionization and selected ion monitoring on a gas chromatograph mass spectrometer (model 5970; Hewlett-Packard Co., Palo Alto, CA). The enrichments of plasma leucine, phenylalanine, and tyrosine were determined by monitoring ions 302 and 303 (leucine), 234 and 239 (phenylalanine), and 466, 468, and 470 (tyrosine) after derivatization to the tertiary butyldimethylsilyl derivatives(6). The plasma enrichment of KIC was determined after derivatization to the O- trimethylsilylquinoxalinol by monitoring ions 232 and 233(7, 8). The final value for all determinations was corrected using an enrichment calibration curve.
13CO2 enrichment. The13 C enrichment in carbon dioxide in blood bicarbonate was measured by isotope ratio mass spectrometry (Finnigan MAT 252, San Jose, CA) after separation of the carbon dioxide by cryogenic distillation in vaccum as previously described(9, 10). The mean enrichment values of three samples taken during enrichment plateau in each subject were used for further analysis.
Substrate and hormone concentrations. Plasma glucose concentrations were determined by the glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH) on all samples obtained; the coefficient of variation was less than 7% during the basal and parenteral nutrition periods. Because only a limited amount of blood was obtained from these premature infants, amino acid concentrations were measured on only one sample during each of the two periods. Plasma insulin concentrations were measured on one sample during each of the two periods by double antibody RIA.
Calculations. Plasma enrichments of leucine, phenylalanine, and tyrosine were used to calculate the rates of appearance of these amino acids. In addition, the leucine rate of appearance was also calculated based upon the enrichment of KIC, the intracellular transamination product of leucine. This precursor was measured because its plasma enrichment has been shown to closely approximate intracellular leucine enrichment(11).
The total rates of appearance of leucine, phenylalanine, and tyrosine were each calculated by measuring the tracer dilution at steady state as modified for stable isotopic tracers(12, 13)Equation where Ra is the rate of appearance of the amino acid, EP is the steady state enrichment of the specific isotope, and I is the rate of tracer infusion.

Endogenous rates of appearance of leucine and phenylalanine (reflecting proteolysis) were calculated by subtracting the rate of exogenous administration of unlabeled leucine or phenylalanine from their measured total rates of appearance(14, 15). Endogenous rates of appearance of leucine were calculated using the unlabeled leucine infusion and the total leucine Ra based on both KIC enrichment and leucine enrichment.
The fraction of leucine oxidized (F) was assessed by determining the amount of infused label that appeared in CO2. The followingequation was used(16): where Vco2 is the carbon dioxide production, and the constant 0.8 represents the fractional recovery of 13CO2(17, 18). Leucine oxidation was then calculated by multiplying the leucine flux by the fraction of leucine flux by the fraction of leucine flux oxidized.

Phenylalanine hydroxylation to tyrosine QPT was calculated as follows(19):Equation where [2H4]Tyr and[2H5]Phe are the isotopic enrichments of the respective tracers in plasma, and IPhe is the infusion rate of tracer phenylalanine (micromoles/kg/h). The expression corrects for the contribution of the phenylalanine tracer infusion to QPT.


Phenylalanine utilization for protein synthesis was calculated by subtracting QPTfrom Phe Ra, because phenylalanine is irreversibly lost either by its degradation pathway via its conversion into tyrosine, or by incorporation into protein(19, 20). Leucine utilization for protein synthesis was calculated by subtracting leucine oxidation from leucine Ra. This calculation was performed using the plasma enrichments of both KIC and leucine.
Statistics. All results are reported as the mean ± SEM. Comparisons were made using the paired t test.
RESULTS
Glucose, insulin, and amino acid concentrations. Plasma glucose concentrations were similar in the basal and parenteral nutrition periods(basal, 4.6 ± 0.2 mmol/L; parental nutrition, 4.8 ± 0.4 mmol/L). Insulin concentrations were also not different between the two periods (basal, 110 ± 15 pmol/L; parenteral nutrition, 115 ± 20 pmol/L). Plasma amino acid concentrations measured during the studies are shown in Table 2. As expected, the plasma concentrations of many amino acids, including all of the essential amino acids, increased during parenteral nutrition. It must be noted, however, that the concentrations of both tyrosine and cysteine significantly decreased during parenteral nutrition; the amino acid solution used provided little tyrosine and no cysteine.
Respiratory calorimetry. The respiratory calorimetry data are reported in Table 3. Both oxygen consumption and energy expenditure were higher during parenteral nutrition (p = 0.06), consistent with a diet-induced thermogenesis response. The RQ declined slightly but significantly, likely as a result of lipid and protein provided during parenteral nutrition.
Leucine, phenylalanine, and tyrosine kinetics. Isotopic steady state was achieved for 13CO2, [1-13C]leucine,[1-13C]KIC, [d5]phenylalanine,[d4]tyrosine, and [d2]tyrosine during both periods as illustrated by Figure 1.
The rates of appearance of leucine (based on both leucine and KIC enrichment), phenylalanine, and tyrosine are reported in Table 4. The rates of appearance leucine and phenylalanine increased significantly during parenteral nutrition. In contrast, the rate of appearance of tyrosine decreased significantly during the parenteral nutrition period.
Leucine and phenylalanine used for protein synthesis is shown in Figure 2. Leucine utilization for protein synthesis increased significantly (≈20%) during parenteral nutrition, as calculated either from leucine or KIC plasma enrichment. Phenylalanine utilization for protein synthesis also significantly increased (≈10%) during the parenteral nutrition period.
The endogenous release of leucine and phenylalanine (reflecting proteolysis) is shown in Figure 3. The endogenous release of leucine based on plasma leucine enrichment significantly decreased(≈10%) during parenteral nutrition. The endogenous release of leucine based on KIC enrichment was slightly but not statistically lower during parenteral nutrition; however, seven of the nine infants exhibited a decrease in the endogenous rate of leucine release (based on KIC enrichment) during parenteral nutrition. Endogenous phenylalanine rate of appearance was significantly lower(≈20%) during parenteral nutrition.
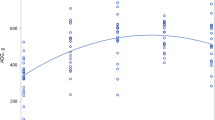
Leucine oxidation and phenylalanine hydroxylation (the irreversible losses of leucine and phenylalanine) is shown in Figure 4. Leucine oxidation increased over 2-fold during parenteral nutrition, based on either the plasma leucine or KIC enrichment. In contrast, phenylalanine hydroxylation during parenteral nutrition was not significantly different from basal values.
Leucine and phenylalanine balance during the basal period and in response to parenteral nutrition is shown in Figure 5. Both leucine and phenylalanine balance increased significantly and changed from negative to positive during parenteral nutrition. Furthermore, the rate of leucine and phenylalanine lost during the basal period and gained during the parenteral nutrition period is consistent with the relative content of leucine and phenylalanine in protein (629 μmol of leucine/g of protein, 280 μmol of phenylalanine/g of protein; 2.2:1 leucine to phenylalanine ratio)(21).
DISCUSSION
In the present study we have examined how the balance and catabolism of leucine and phenylalanine in premature infants are affected by parenteral nutrition. Although providing parenteral nutrition shifts leucine and phenylalanine balance from negative to positive, leucine and phenylalanine catabolism are differently affected by parenteral nutrition. Although phenylalanine irreversible losses are unchanged from basal values during parenteral nutrition, leucine losses are doubled. In addition, the acute anabolic changes produced by parenteral nutrition in premature infants were evaluated. As reflected by leucine and phenylalanine kinetics, parenteral nutrition produces significant increases in protein synthesis; the data also suggest that these increases in protein synthesis are accompanied by reductions in proteolysis.
The most important aspect of the present study was the simultaneous determination of the catabolism of two essential amino acids to reflect their relative supply in parenteral nutrition. It must be noted, however, that the catabolic pathway of phenylalanine has been a particular issue in premature newborns. Hydroxylation to tyrosine represents the catabolic pathway of phenylalanine; the ability of the premature infant to actively convert phenylalanine to tyrosine has long been questioned(22–25). It is clear from the present study and other investigations(4, 26) that premature infants have the capacity for phenylalanine hydroxylation. In fact, the phenylalanine hydroxylation rates (micromoles/kg) measured in the premature infants of this study are 2-fold higher than those measured in adults(19).
Although active rates of phenylalanine hydroxylation were present in the basal state, no increases in these rates occurred in response to parenteral nutrition. In contrast, leucine oxidation (the irreversible loss of leucine by catabolism) increased 2-fold over basal rates when parenteral nutrition was administered. One potential explanation for this increase in leucine and not phenylalanine catabolism during parenteral nutrition is that phenylalanine hydroxylation rates in premature infants may already be maximal in the basal state. However, this seems unlikely in view of our recent study in extremely premature infants (≈26-wk gestation), which measured basal phenylalanine hydroxylation rates 50% higher than those determined in the ≈33-wk gestation premature infants of the present study(4). Furthermore, extremely premature infants of 26-wk gestation and 29-wk gestation did significantly increase phenylalanine hydroxylation in response to parenteral nutrition(4, 26).
Another potential explanation for the substantial differences in the irreversible losses of leucine and phenylalanine during parenteral nutrition is that the leucine being provided by the amino acid solution is in excess of that required for net protein accretion, but phenylalanine is not. Support for this possibility is provided by the net balance of leucine and phenylalanine in the basal state and in response to parenteral nutrition (Fig. 5). In the basal state, the net balance of both essential amino acids is negative as expected; leucine losses are approximately twice that of phenylalanine losses, consistent with their relative content in protein(21). In response to parenteral nutrition, both essential amino acid balances are positive, but the 2:1 ratio of leucine to phenylalanine balance is maintained. This 2:1 positive balance of leucine to phenylalanine during parenteral nutrition is maintained even though the amino acid solution provides leucine and phenylalanine in a 4:1 ratio. The 2:1 net balance of leucine to phenylalanine is achieved by the substantial increase in the leucine oxidation rate without a change in the rate of phenylalanine hydroxylation.
These leucine and phenylalanine balance data do not clearly resolve whether the amino acid solution in parenteral nutrition provides leucine in excess or phenylalanine in limited amounts. There is, however, reason to believe that phenylalanine administration may be the limiting factor. The amount of both leucine and phenylalanine contained per g of neonatal amino acid solution is similar to the amount of leucine and phenylalanine contained per g of protein in premature formula or human milk(27). However, because of the extremely limited solubility of tyrosine, the amount of tyrosine contained in the neonatal amino acid solution is approximately 10% of that contained in a similar amount of premature formula or human milk(26). The phenylalanine supplied by the amino acid solution therefore must provide not only sufficient phenylalanine for protein accretion, but also sufficient tyrosine (via phenylalanine hydroxylation). For this reason, it seems likely that the phenylalanine supply (more accurately the combined phenylalanine/tyrosine supply) may be a limiting factor in protein accretion during parenteral nutrition as provided in the present study to this group of premature infants. However, we must note that the phenylalanine supply may not be the limiting factor in all premature infants. As we have previously noted, less mature premature infants increase phenylalanine hydroxylation in response to parenteral nutrition(4, 26). One might speculate that the supply of other amino acids (i.e. cysteine) or total calories may be important limiting factors in this population.
Although it is possible phenylalanine may be limiting in some currently available neonatal amino acid solutions, it is unclear at present how or if these solutions should be altered. Although increasing the amount of phenylalanine is a possible strategy, it is uncertain to what extent additional phenylalanine can be converted to tyrosine by premature infants to adequately compensate for the minimal tyrosine supply. Alternatively, a more soluble form of tyrosine might be added to the amino acid solution; some neonatal amino acid formulations contain the soluble tyrosine derivative N- acetyltyrosine. However, because N-acetyltyrosine has limited bioavailability(28), it is unclear whether this particular tyrosine derivative is a useful exogenous tyrosine source. Although other tyrosine derivatives appear to be more bioavailable (i.e. glycyltyrosine)(29, 30), these derivatives have not been evaluated in premature infants. Further extensive studies must be undertaken before changes in neonatal amino acid formulations can be recommended.
In addition to assessing the catabolism and balance of leucine and phenylalanine, the present study also provides information about how parenteral nutrition acutely alters protein kinetics in premature infants. Parenteral nutrition clearly increases whole body protein synthesis above already high basal rates as reflected by both the essential amino acid tracers. This result provides the strongest evidence to date that changes in protein synthesis are important in achieving protein accretion during parenteral nutrition in premature infants; this finding is consistent with other studies carried out in premature infants using a single amino acid tracer (leucine or phenylalanine) to assess whole body protein synthesis(4, 31, 32).
It is interesting to note that, regardless of the tracer model, parenteral nutrition produced a reduction in proteolysis in these premature infants which was intermediate between 26-wk premature infants and full-term normal newborns studied under a protocol identical to that of the present study. Using an average of the tracer data, parenteral nutrition reduced proteolysis by 1% in 26 wk gestation premature infants, by 11% in the 32-wk gestation premature infants of the present study, and by 19% in full-term normal newborns(4). These results support the concept that parenteral nutrition effects changes in proteolysis along a developmental continuum.
In summary, the present study demonstrates that premature infants respond to parenteral nutrition with an acute increase in whole body protein synthesis as well as an intermediate reduction in proteolysis. During parenteral nutrition, leucine oxidation increases 2-fold, whereas phenylalanine hydroxylation is unchanged, suggesting a possible limitation in the phenylalanine/tyrosine supply. If limiting, additional phenylalanine and/or tyrosine may reduce leucine oxidation and increase leucine accretion during parenteral nutrition. This hypothesis remains to be proven.
Abbreviations
- KIC:
-
α-ketoisocaproic acid
References
Pereira G 1995 Nutritional care of the extremely premature infant. Clin Perinatol 22: 61–75
Heird W, Gomez M 1992 Parenteral nutrition. In: Tsang R(ed) Nutritional Needs of the Preterm Infant. Williams & Wilkins, Baltimore, pp 255–242
Zello GA, Wykes LJ, Ball RO, Pencharz PB 1994 Recent advances in methods of assessing dietary amino acid requirements for adult humans. J Nutr 125: 2907–2915
Denne SC, Karn CA, Ahlrichs JA, Dorotheo AR, Wang J, Liechty EA 1996 Proteolysis and phenylalanine hydroxylation in response to parenteral nutrition in extremely premature and normal newborns. J Clin Invest 97: 746–754
Denne S, Kalhan S 1987 Leucine metabolism in human newborns. Am J Physiol 253:E608–E615
Schwenk W, Berg P, Beaufrere B, Miles J, Haymond M 1984 Use of t-butyldimethylisilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem 141: 101–109
Denne SC, Karn C, Wang J, Liechty E 1995 Effect of intravenous glucose and lipid on glucose production and proteolysis in normal newborns. Am J Physiol 269:E361–E367
Wolfe R 1992 Radioactive and stable isotope tracers in biomedicine. In: Principles and Practice of Kinetic Analysis. Wiley-Liss, New York, pp 471
Denne SC, Kalhan SC 1986 Glucose carbon recycling and oxidation in human newborns. Am J Physiol 251:E71–E77
Kalhan SC, Ricanati ES, Tserng KY, Saving SM 1983 Glucose turnover in chronic uremia: increased recycling with diminished oxidation of glucose. Metabolism 32: 1155–1162
Schwenk W, Beaufrer B, Haymond M 1985 Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol 249:E646–E650
Steele R 1959 Influence of glucose loading and of injected insulin on hepatic glucose output. Proc NY Acad Sci 82: 420–430
Tserng K, Kalhan S 1983 Calculation of substrate turnover rate in stable isotope tracer studies. Am J Physiol 245:E308–E311
Castellino P, Luzi L, Simonson D, Haymond M, deFronzo R 1987 Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. J Clin Invest 80: 1784–1793
Flakoll P, Kulaylat M, Frexes-steed M, Hourani H, Brown L, Hill J, Abumrad N 1989 Amino acids augment insulin's suppression of whole body proteolysis. Am J Physiol 257:E839–E847
Denne SC, Karn CA, Liechty EA 1992 Leucine kinetics after a brief fast and in response to feeding in premature infants. Am J Clin Nutr 56: 899–904
Allsop JR, Wolfe RR, Burke JF 1978 Tracer priming the bicarbonate pool. J Appl Physiol 45: 137–139
Van Aerde JEF, Sauer PJJU, Pencharz PB, Canagarayar U, Beesley J, Smith JM, Swyer PR 1985 The effect of energy intake and expenditure on the recovery of 13CO2 in the parenterally fed neonate during a 4-hour primed constant infusion of HAH 13CO3 . Pediatr Res 19: 806–810
Thompson G, PF P, Merritt H, Ford G, Read M, Cheng K, Halliday D 1989 Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol 256:E631–E639
Nair K, Ford G, Ekberg K, Fernqvist-Forbes E, Wahren J 1995 Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest 95: 2926–2937
Munro HN, Fleck A 1969 Analysis of tissues and body fluids for nitrogenous constituents. In: Munro HN (ed) Mammalian Protein Metabolism. Academic Press, New York, pp 423–525
Snyderman SE 1971 The protein and amino acid requirements of the premature infants. In: Visser HKA, Toreistra JA (ed) Nutricia Symposium: Metabolic Processes in the Fetus and Newborn Infant. Stenfert Kroese, Leiden, Netherlands, pp 128–141
Heird W, Kashyap S 1992 Protein and amino acid requirements. In: R Polin, W Fox (eds) Fetal and Neonatal Physiology, Vol I. WB Saunders, Philadelphia, pp 450–452
Uauy R, Greene H, Heird W 1993 Conditionally essential nutrients. In: R Tsang (ed) Nutritional Needs of the Preterm Infant. Williams& Wilkins, Baltimore, pp 267–280
Rassin D 1994 Essential and non-essential amino acids in neonatal nutrition. In: Raiha N (ed) Protein Metabolism during Infancy, Vol 33. Raven Press, New York, pp 183–192
Kilani RA, Cole FS, Bier DM 1995 Phenylalanine hydroxylase activity in preterm infants: is tyrosine a conditionally essential amino acid?. Am J Clin Nutr 61: 1218–1223
Atkinson SA 1992 Nitrogen sources for the newborn In: Polin R, Fox W (eds) Fetal and Neonatal Physiology. WB Saunders, Philadelphia, pp 443–450
Christensen ML, Helms RA, Veal DF, Boehm KA, Storm MC 1993 Clearance of N-acetyl-L-tyrosine in infants receiving a pediatric amino acid solution. Clin Pharmacol 12: 606–609
Albers S, Wermerman J, Stehle P, Vinnars E, Furst P 1988 Availability of amino acids supplied intravenously in healthy man as synthetic dipeptides: kinetic evaluation of L-alanyl-L-glutamine and glycyl-L-tyrosine. Clin Sci 75: 463–468
Wykes LJ, House JD, Ball RO, Pencharz PB 1994 Amino acid profile and aromatic amino acid concentration in total parenteral nutrition. Effect on growth, protein metabolism and aromatic amino acid metabolism in the neonatal piglet. Clin Sci 87: 75–84
Mitton SG, Garlick PJ 1992 Changes in protein turnover after the introduction of parenteral nutrition in premature infants: comparison of breast milk and egg protein-based amino acid solutions. Pediatr Res 32: 447–454
Rivera A, Bell EF, Bier DM 1993 Effect of intravenous amino acids on protein metabolism of preterm infants during the first three days of life. Pediatr Res 33: 106–110
Acknowledgements
The authors thank Katherine Lee for her technical assistance, and Billie Riley and Patricia Fox for help in the preparation of this manuscript.
Author information
Authors and Affiliations
Additional information
Supported in part by the National Institutes of Health Grants M-01RR750, P-60DK20542, and RO1 HD-29153, the James Whitcomb Riley Memorial Association, and by Bristol-Meyers Squibb, Inc.
Rights and permissions
About this article
Cite this article
Clark, S., Karn, C., Ahlrichs, J. et al. Acute Changes in Leucine and Phenylalanine Kinetics Produced by Parenteral Nutrition in Premature Infants. Pediatr Res 41, 568–574 (1997). https://doi.org/10.1203/00006450-199704000-00019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199704000-00019
This article is cited by
-
Total Parenteral Nutrition-associated Cholestasis: Prematurity or Amino Acids?
Journal of Perinatology (2003)