Abstract
The influence of long chain polyunsaturated fatty acids (LCP) in formula feeds on lipid peroxidation and antioxidants was studied in 35 healthy preterm infants (gestational age 30-35 wk) during the first 6 postnatal weeks. Infants received a preterm formula supplemented with n-3 LCP (LCP group,n = 13), or standard preterm formula (NO-LCP group, n = 15); 7 infants fed human milk served as a reference group. With LCP supplementation, erythrocyte C22:6n-3 levels were stable; without supplementation, the levels declined (difference p < 0.001). LCP supplementation did not decrease vitamin E or C levels, or increase lipid peroxidation products (thiobarbituric acid-reactive substances) in plasma. In erythrocytes, LCP supplementation did not markedly influence the reduced/oxidized glutathione ratio; however, the susceptibility to H2O2-induced oxidative stress was reduced. Our results suggest that healthy preterm infants are able to cope with any extra peroxidative stress produced by n-3 LCP supplementation. However, these findings might not be generally applicable to other formulas containing LCP supplements.
Similar content being viewed by others
Main
LCP docosahexaenoic acid C22:6n-3 and arachidonic acid C20:4n-6 are important components of the cell membranes in brain and retina(1). Decreased exogenous supply of C22:6n-3 in preterm feeds may decrease visual acuity and delay psychomotor development(1–4). The European Society for Pediatric Gastroenterology recommended that preterm formulas be supplemented with LCP(5), and various formulations are now commercially available. However, polyunsaturated fatty acids are highly susceptible to ROS damage(6, 7), and increased dietary intake might increase the susceptibility of the preterm infant to oxygen toxicity. ROS play an important role in the pathogenesis of diseases such as bronchopulmonary dysplasia and necrotizing enterocolitis(8), and the possible disadvantages of using LCPs must be assessed. Although the effect of increased dietary LCP on oxidative damage is being investigated extensively in vitro and in adults(9–12), it has received little attention in the preterm infant(13, 14).
We studied the effect of adding n-3 LCP to formula feeds on ROS metabolism in healthy preterm infants. Lipid peroxidation and antioxidants were measured in plasma and erythrocytes, and the in vitro ability of erythrocytes to withstand hydrogen peroxide-induced stress was assessed. The results were compared with those found in infants fed human milk.
METHODS
This study was approved by the Scientific Committee of the Department of Pediatrics of the University Hospital of Leiden and the Ethical Committee of the Juliana Children Hospital, The Hague. Informed parental consent was obtained.
Patients
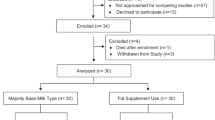
Fifty-two preterm infants entered the trial. Seventeen infants were withdrawn in the 1st wk, and their measurements were not analyzed: 16 infants changed from human milk to formula feeds or vice versa (see the following nutrition section), and one baby fed the control formula without LCP developed necrotizing enterocolitis. Table 1 shows the clinical characteristics of the remaining 35 preterm infants (gestational age 30-35 completed weeks), who completed the 6-wk study period. Four of these infants, two infants fed human milk and one infant fed each formula, were withdrawn after d 21 because of changes in the supply of their mothers' milk; their measurements up to this period are included in the results.
In an attempt to limit other factors influencing ROS metabolism(e.g. neutrophil activity due to infection or oxygen therapy) we studied healthy preterm infants. None of the infants had birth asphyxia (Apgar score at 5 min >6, no resuscitation), sepsis, or severe respiratory distress (supplemental oxygen <8 h, fractional concentration of inspired air (Fio2) ≤ 25%, respiratory support <48 h). All infants started enteral nutrition on d 1, and none required parenteral nutrition. No infant developed anemia requiring erythrocyte transfusion. No infant developed bronchopulmonary dysplasia or retinopathy of prematurity. All infants, followed up until the age of 2 y, were developing normally except one child. This child, on the control formula without LCP, with disturbed neuromotor development was clinically well during the feeding study and his biochemical measurements did not differ from his cohort.
Nutrition
All mothers were encouraged to breast-feed. If they chose not to breast-feed or supplementary feeding was required, the infants were given one of the two formula feeds in a random double blind manner. Feeds started within 24 h after birth and incrementally increased to 150 mL/kg/d by d 7. Depending on the success of breast-feeding, the infants were later assigned to a formula or breast-feeding group. Infants were assigned to the human milk group if they received >90% of their intake as human milk at the end of 2nd wk and to one of the formula groups if they received >90% of their intake as formula at the end of the 2nd wk. Infants receiving other proportions of formula and breast milk were excluded (n = 16). No iron or vitamin E or C supplements were given; vitamin A and D (retinol palmitate, 1800 IU/d, and cholecalciferol, 600 IU/d) were given from d 22.
Fifteen infants received a formula, not containing LCP, designated as NO-LCP. This is a regular preterm formula, which was commercially available during the study period (Nenatal®, Nutricia BV, Zoetermeer, The Netherlands). Thirteen infants received a formula containing LCP, designated as LCP. This was identical to NO-LCP except for changes in the lipid composition (Table 2): linoleic acid was lowered 5% and replaced by n-3 LCP from fish oil (0.5% eicosapentaenoic acid and 0.5% docosahexaenoic acid) and oleic acid (4%). α-Linolenic acid concentration was similar in the two formulas, and arachidonic acid was present in a concentration of less than 0.05%. Due to these changes the total amount of polyunsaturated fatty acids was lower in the LCP formula; however, the number of double bonds (expressed as the active hydrogen (H) content) was similar (Table 2). Both formulas had the same vitamin E content, which resulted in a higher vitamin E/PUFA ratio (vitamin E/PUFA IU/mmol: NO-LCP 0.67, LCP 0.88), but a similar vitamin E to active H content in the LCP formula (vitamin E/active IU/number: NO-LCP 0.055, LCP 0.057). Seven infants were fed on their own mothers' milk (human milk): the milk was administered fresh, or kept at either 4 °C for up to 24 h or at -20 °C until used.
Measurements
Heparinized blood (1.5 mL) was collected by venipuncture before feeding on d 1 (12-24 h), 3 (60-96 h), 7 (6-8 d), 14 (13-16 d), 21 (19-24 d), and 42(39-49 d) postpartum from formula groups and on d 1, 21, and 42 from the human milk group. Samples were prepared for analysis within 30 min after collection: after centrifugation (750 × g, 10 min), the erythrocytes were washed twice with PBS. A sample of the red cells was used immediately for the glutathione measurements and hydrogen peroxide-induced lipid peroxidation; the remaining erythrocytes and plasma were stored for the other analyses (-70°C, under argon). Preliminary studies showed that frozen storage did not influence the results.
Erythrocyte fatty acid composition. The erythrocyte fatty acid composition was measured by a standard method(15) without prior lipid extraction. Two hundred microliters of erythrocyte suspension were transesterified at 100 °C for 1 h after the addition of 2 mL of methanol/benzene (4/1, vol/vol) and 0.2 mL of acetylchloride. After neutralization with 5 mL of 6% H2CO3 and centrifugation, the benzene phase was dried under nitrogen, and the residue was dissolved in 50μL of n-hexane. The fatty acid methylesters were analyzed on a Hewlett-Packard 5890A gas liquid chromatograph, with a fused silica column (CP Sil 8CB, Chrompack, Middelburg, The Netherlands) using the operating conditions previously described by Onkenhout et al.(16).
The susceptibility of the polyunsaturated fatty acids in the erythrocyte membrane to peroxidation was assessed by calculating the total active H content. The average number of bisallylic hydrogens (active H atoms) per molecule of fatty acid was as follows. From each individual fatty acid the active H content was calculated: 2 × [number of double bounds in the fatty acid - 1], and multiplied by the sum of the mol% of the fatty acid. Then the outcomes of all of the individual fatty acid active H content results were added, and this resulted in the total active H content(17).
Vitamin E and vitamin E/total-lipid ratio in plasma. Plasma with added SDS (50/50, vol/vol) was mixed with ethanol containing tocopherol acetate as an internal standard. After addition of heptane and centrifugation, the supernatant was removed, dried under nitrogen, and resuspended in 150μL of the mobile phase(18). Analysis was performed by HPLC, equipped with a spherisorb ODS-2 3-μm column (Altech, Breda, The Netherlands) with fluorometric detection. The interassay coefficient of variation of the vitamin E measurement was 6.2%. For the plasma vitamin E/total-lipid ratio, the total lipid was calculated as the sum of the concentrations of cholesterol, triglycerides, and phospholipids. Cholesterol and triglycerides were measured on the SMACII automatic analyzer (Technicon Instruments, Tarrytown, NY) and phospholipids according to Folch et al.(19).
Plasma vitamin C. Total vitamin C in plasma was transformed to its oxidized form by enzymatic oxidation. The oxidized product was condensed with o-phenylene diamine to produce quinoxaline derivative, which was measured by HPLC, equipped with a hypersil BDS 5-μm column (Altech, Breda, The Netherlands) with fluorimetric detection(20). The interassay coefficient of variation of this measurement was 3.1%.
Plasma TBARS. Plasma TBARS were measured fluorometrically(21, 22). The interassay coefficient of variation of this test was 3.5%. The measured TBARS value was corrected for the influence of plasma bilirubin, subtracting the value (0.034 × bilirubin concentration)(23). Bilirubin was measured on the SMACII automatic analyzer (Technicon Instruments, Tarrytown, NY).
Erythrocyte GSH/GSSG. These indices of oxidative stress were measured by flow injection analysis using an enzymatic recycling reaction(24). The interassay coefficient of variation of this test was 2.5%.
Lipid peroxidation of erythrocytes (erythrocytes TBARS). Fresh erythrocytes were exposed to H2O2 to assess their sensitivity to peroxidative stress(25). A 2.5% hematocrit erythrocyte suspension in 1 mM sodium azide PBS was exposed to 10 mM H2O2 and incubated (37 °C, shaking water bath). Before addition and after 20 min of incubation of H2O2, the TBARS were measured by fluorimetry(22) as an index of lipid peroxidation(26). The interassay coefficient of variation of this test was 6%. This test was also performed in five healthy nonsmoking adults to establish reference values.
Statistics
The data, presented as mean ± SD, were tested for normality. To test for differences on d 1, ANOVA was performed. To test for the effect of time, diet, and the interaction between time and diet in the NO-LCP and LCP group repeated measures analyses of variance (mixed model MANOVA) were used. The human milk group was excluded from these analyses because of missing paired values. The results of the human milk-fed infants were compared only on d 42 with the formula groups (ANOVA). The correlation of TBARS production after exposure to H2O2 with the active H content and unsaturated fatty acids on d 42 was evaluated by the Pearson correlation test. p values <0.05 were regarded significant; in multiple pairwise comparisons, Scheffé correction was applied. Analyses were executed with the Statistical Package for Social Sciences (SPSS)/PC+ version 3.0 statistical programming language (SPSS, Inc., Chicago).
RESULTS
Clinical Measurements
At birth there were no significant differences in antenatal factors, gestational age, weight, or sex distribution (Table 1). The head circumference was larger in the NO-LCP group compared with the LCP group. Postnatal increases in weight, length, and head circumference were normal in all infants; however, the human milk group showed less gain in weight and head circumference than the formula groups (p < 0.05).
Biochemical Measurements
The trends in the measurements of the NO-LCP and LCP group were compared on all postnatal days (MANOVA), but the human milk group were included in the comparison only on d 42 (ANOVA, see “Statistics”).
Erythrocyte fatty acid composition. Table 3 shows the fatty acid composition of the erythrocytes at d 1 and 42. The other postnatal days are not shown but were included in the statistical analyses.
Postnatal changes in monounsaturated fatty acids. In both formula groups the level of C16:1 decreased, and the level of C18:1 increased postnatally (p < 0.001); these trends did not differ between the two formulas. On d 42, comparison of the formulas and human milk groups showed that there were no differences in the C16:1 or C18:1 values.
Postnatal changes in n-6 fatty acids. In both formula groups the levels of C18:2n-6 and C18:3n-6 increased, and the level of C20:4n-6 decreased postnatally (p < 0.001,p < 0.01, and p < 0.001, respectively); these trends did not differ. On d 42 there were no differences between the formula groups and the human milk group.
Postnatal changes in n-3 fatty acids:. In both formula groups C18:3 levels increased postnatally (p < 0.01); these trends did not differ. On d 42 levels were higher in the NO-LCP group and LCP group compared with the human milk group (p < 0.05). In the formula groups C20:5n-3 fell in the NO-LCP group and rose in the LCP group, these changes were not significant, but their opposite trends resulted in a significant different postnatal course between the two formula groups(p < 0.01). On d 42, values did not differ between the feeding groups. C22:5n-3 levels rose postnatally in the LCP and NO-LCP groups (p < 0.001); the pattern was similar, and the groups did not differ on d 42. The level of C22:6 remained stable in the LCP group and fell in the NO-LCP group (difference, p < 0.001). On d 42, levels were higher in the LCP group compared with the NO-LCP group (p < 0.01).
Active H content of erythrocyte membranes. The active H contents of the erythrocyte membranes are shown in Table 3. Active H declined in the formula groups postnatally (p < 0.001); these trends did not differ. Day 42 levels did not differ between the feeding groups.
Postnatal changes in markers of oxidant stress. In both formula groups vitamin E levels increased postnatally (both p < 0.001): vitamin E μmol/L mean (±SD) NO-LCP group: d 1, 10.3 (2.3); d 42, 32.7 (15.2); and LCP group: d 1, 7.3 (2.2); d 42, 23.9 (14.5). The trend(group and time interaction) between the LCP and NO-LCP group did not differ. In the human milk group vitamin E levels also rose: d 1, 6.4 (2.4); d 42, 19.6(8.6); d 42 levels were the same in formula and human milk groups.
In both formula groups vitamin E/total-lipid ratio increased postnatally(both p < 0.001): vitamin E/total-lipid ratio μmol/mmol mean(SD) NO-LCP group: d 1, 2.2 (0.4); d 42, 5.8(2.0); and LCP group: d 1, 2.0(0.3); d 42, 4.2 (2.5). The trend between the LCP and NO-LCP group did not differ. In the human milk group the vitamin E/total-lipid ratio also rose: d 1, 1.6 (0.7); d 42, 3.1 (1.5); d 42 levels were the same in formula and human milk groups. None of the infants had evidence of a low vitamin E/total-lipid ratio (<0.6 μmol/mmol) at any time during the study.
In both formula groups vitamin C levels increased postnatally (bothp < 0.001): vitamin C, μmol/L mean (±SD): NO-LCP group, d 1, 65 (33); d 42, 100 (28); and LCP group, d 1, 63 (28); d 42, 102 (32). The trend between the LCP and NO-LCP group did not differ. In the human milk group vitamin C levels also rose: d 1, 63(30); d 42, 67 (37); d 42 levels were the same in formula and human milk groups.
Figure 1 shows the postnatal changes in plasma TBARS levels. In both formula groups plasma TBARS levels remained stable and did not differ between these groups. On d 42 these levels did not differ between any of the feeding groups.
Figure 2 shows the postnatal changes in erythrocyte GSH/GSSG ratio. The ratio remained stable in the LCP group, but increased in the NO-LCP group (p < 0.01); these trends differed significantly(p < 0.05). However, the d-42 comparison of the three feeding groups did not show any significant differences.
Postnatal changes in GSH/GSSG ratio in the different feeding groups; mean (±SD). The trends in the measurements of the NO-LCP and LCP group were compared on all postnatal days; the human milk group trend was included in the comparison only on d 42. **p < 0.01 significant postnatal changes in the indicated group using MANOVA.
Figure 3 shows the in vitro erythrocyte TBARS production after exposure to H2O2. In both formula groups the erythrocyte TBARS production after exposure to H2O2 decreased over the 42 d (p < 0.001); the trends did not differ. Day-42 levels were lower in the LCP group compared with the NO-LCP group and the human milk group (p < 0.05 and p < 0.001, respectively); the human milk group levels were higher than the NO-LCP group(p < 0.05).
Postnatal changes in erythrocyte TBARS production after exposure to H2O2 in the different feeding groups; mean(±SD). The trends in the measurements of the NO-LCP and LCP groups were compared on all postnatal days; the human milk group trend was included in the comparison only on d 42. ***p < 0.001 significant postnatal changes in the indicated group using MANOVA. A, B, andC = significant difference between the indicated groups on d 42 using ANOVA.
On d 42, in the formula groups there was no correlation between erythrocyte TBARS production and the level of the individual mono- or polyunsaturated fatty acids.
DISCUSSION
LCPs, which are increasingly added to infant feeding formulas(2, 27), are prone to lipid peroxidation, and their effect on ROS formation in the preterm infant has received little attention(13, 14). In adults supplementation of fatty acids gives conflicting results: linoleic acid in parenteral lipid emulsion increases lipid peroxidation products(28), and fish oil supplements either increase or decrease oxidative sensitivity(10–12). In preterm infants results are also not conclusive: linoleic acid in parenteral lipid emulsions increases lipid peroxidation products(29) and increases the incidence of bronchopulmonary dysplasia and overall mortality(30, 31), whereas enteral LCP supplements lowered TBARS production(13) but did not influence vitamin E and A concentrations or membrane fluidity(14).
In this study we evaluated the effect of n-3 LCP supplementation on the membrane composition of the erythrocyte and its influence on lipid peroxidation. n-3 LCP supplementation did produce changes in erythrocyte membrane composition; however, there was no evidence of increased susceptibility to lipid peroxidation in plasma and erythrocytes.
The LCP formula contained a lower total amount of polyunsaturated fatty acids than did the NO-LCP formula. However, these were longer chain polyunsaturated fatty acids, and therefore the total amount of potentially peroxidizable double bonds (active hydrogen) was similar (see“Methods”).
As previously shown, this study also showed that the dietary intake of fatty acids was reflected in the membrane composition of the erythrocyte. Our infants fed formula supplemented with 0.5% C22:6n-3, maintained their C22:6n-3 levels on the same level as those on human milk(2, 13, 27). Infants fed the unsupplemented formula did not maintain levels of n-3 LCP comparable to human milk-fed infants, despite an intake of C18:3n-3 considered to be adequate (1.6%)(2, 13). Significant reductions ofn- 6 LCP have been reported after supplementation with C22:6n-3 and C20:5n-3(27): in our study C20:4n-6 fell, but their was no significant difference between the formulas. This might be due to the shorter duration of our study(13, 14, 27). In the LCP formula, linoleic acid was partly replaced by oleic acid. However, this did not produce a significantly higher erythrocyte oleic acid concentration on d 42. These changes in erythrocyte LCPs did not produce a significant difference in the active hydrogen content of the erythrocytes in the three feeding groups on d 42.
TBARS were used as indicators of lipid peroxidation in plasma and erythrocytes. LCP supplementation did not elevate the TBARS. This could be interpreted as no increase in lipid peroxidation. Unfortunately, we did not use the more recently developed HPLC method, which removes interference from bilirubin and endoperoxides (eiconasoids). As discussed in“Methods,” we did correct for bilirubin; however, eiconasoids levels could have influenced our results(32).n- 3 LCP inhibits n-6 elongation and thus the production of arachidonic acid and eiconasoids(27); therefore, any increase in TBARS due to increased lipid peroxidation could have been counteracted by a concurrent decrease in eiconasoid production.
Sacrificial antioxidants, e.g. vitamin E and C, are consumed when oxidative stress increases. In adults fed fish oil, plasma vitamin E levels fall if their diet is not supplemented with vitamin E(11). In our study there were no differences in plasma vitamin E measurements between LCP and NO-LCP groups. The vitamin E intake was high, but similar in both formulas. Due to their different fat blends this resulted in a high vitamin E/PUFA ratio in the LCP formula. At first sight this suggests a better protection to oxidative stress; however, the vitamin E to active hydrogen content was similar. The number of active hydrogen atoms is probably a better indicator of susceptibility to lipid peroxidation than the molar concentration of LCP. The influence of fish oil supplementation on plasma vitamin C does not appear to have been studied in adults. However,in vitro vitamin C is consumed before vitamin E, when lipid peroxidation is induced(33). Therefore, this might be a more sensitive marker of clinical oxidative stress(34). In our study vitamin C levels rose similarly postnatally in both formula groups, suggesting that there was no increased oxidative stress in the LCP group. In the human milk-fed infants the levels did not rise, and this may have been the result of a lower vitamin C intake due to storage of the human milk(35).
The GSH/GSSG ratio in erythrocytes is a sensitive marker of oxidative stress in vitro(36) and in vivo(37, 38). In our study, although the ratio increased postnatally in the NO-LCP group in contrast to the LCP group, there were no differences between the three feeding groups on d 42. Therefore, this marker also did not provide convincing evidence of increased oxidative stress in the LCP-fed infants.
As well as the above in vivo responses, we studied in vitro the erythrocyte production of TBARS due to H2O2-induced stress. Erythrocyte TBARS production decreased with increasing postnatal age in both formula groups and was lower on d 42 in the LCP supplemented group. The postnatal decrease could have been related to the increase in vitamin E levels, although this does not explain the differences between the two groups which had similar vitamin E levels. An alternative explanation for the difference in erythrocyte TBARS production could be differences in the susceptibility of the erythrocyte lipids to peroxidation. However, there were no differences in their total number of peroxidizable double bonds (active H content), which reflected the composition of the formulas. Similarly the erythrocyte monounsaturated fatty acid content (e.g. C18:1) did not differ in both groups, despite a higher intake in the LCP group. Thus inhibition of lipid peroxidation by monounsaturated fatty acids, which has been shown in cell cultures and human studies(39, 40), also does not explain these findings. Another factor could have been increased antioxidant enzyme activity in the LCP group. In adult studies fish oil supplementation increases glutathione peroxidase activity(10). We unfortunately did not measure erythrocyte antioxidant enzymes in this study, and further studies are needed to elucidate this factor in the neonatal period.
Seven infants fed human milk served as a reference group, and the fatty acid composition of the erythrocytes was similar to that reported in previous studies(2). The TBARS production after H2O2-induced stress was higher than in both formula groups. Their is no clear explanation for this. The active H content in erythrocytes was not significantly higher, and the levels of vitamin E and C and the GSH/GSSG ratio were similar to the formula groups. Other differences due to human milk feeding, e.g. antioxidant enzyme activity, might play a role.
Clinical implications. Modifications of the fatty acid composition of enteral and parenteral diets to improve growth and development may modify the sensitivity of the newborn baby to oxidative stress. Our study provides preliminary evidence that in the well baby n-3 LCP supplementation does not increase lipid peroxidation. However, the susceptibility of the infant to oxidative stress when on a LCP supplemented feed may be influenced by other pro- and anti-oxidant factors and the composition of the LCPs added. In our study the high vitamin E content in both formulas and the addition of C18:1 in the LCP formula might have prevented lipid peroxidation. Furthermore, in this study the LCP was raised without increasing the amount of active hydrogen (i.e. linoleic acid was replaced by LCP and also oleic acid).
The erythrocyte may not reflect the changes in other tissues such as lung, brain, and intestine. Nevertheless, these findings suggest ways that might be used in developing a safe formula for ill preterm babies, who are undergoing greater oxidative stress.
Abbreviations
- LCP:
-
long chain polyunsaturated fatty acids
- GSH/GSSG:
-
reduced/oxidized glutathione
- TBARS:
-
thiobarbituric acid reactive substances
- ROS:
-
reactive oxygen species
- PUFA:
-
polyunsaturated fatty acid
- ANOVA:
-
analysis of variance
- MANOVA:
-
multivariate analysis of variance
References
Crawford MA 1993 The role of essential fatty acids in neural development: implications for perinatal nutrition. Am J Clin Nutr 57 ( suppl): 703S–710S.
Desci T, Koletzko B 1994 Polyunsaturated fatty acids in infant nutrition. Acta Paediatr 395 ( suppl): 31–37.
Bjerve KS, Brubakk AM, Fougner KJ, Johnsen H, Midthjell K, Vik T 1993 ω-3 fatty acids: essential fatty acids with important biological effects, and serum phospholipid fatty acids as markers of dietaryω-3 fatty acid intake. Am J Clin Nutr 57 ( suppl): 801S–806S.
Hoffman DR, Birch EE, Birch DG, Uauy RD 1993 Effects of supplementation with ω-3 long-chain polyunsaturated fatty acids on retinal and cortical development in premature infants. Am J Clin Nutr 57 ( suppl): 807S–812S.
ESPGAN Committee on Nutrition: Committee report 1991 Comment on the content and composition of lipids in infant formulas. Acta Paediatr Scand 80: 887–896.
Halliwell B, Chirico S 1993 Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57 ( suppl): 715S–725S.
Esterbauer H 1993 Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr 57 ( suppl): 779S–786S.
Saugstad OD 1990 Oxygen toxicity in the neonatal period. Acta Paediatr Scand 79: 881–892.
Garrido A, Garrido F, Guerra R, Valenzuela A 1989 Ingestion of high doses of fish oil increases the susceptibility of cellular membranes to the induction of oxidative stress. Lipids 24: 833–835.
Olivieri O, Negri M, De Gironcoli M, Bassi A, Guarini P, Stanzial AM, Grigolini L, Ferrari S, Corrocher R 1988 Effects of dietary fish oil on malondialdehyde production and glutathione peroxidase activity in hyperlipidaemic patients. Scand J Clin Lab Invest 48: 659–665.
Nair PP, Judd JT, Berlin E, Taylor PR, Shami S, Sainz E, Bhagavan HN 1993 Dietary fish oil-induced changes in the distribution ofα-tocopherol, retinol, and β-carotene in plasma, red blood cells, and platelets: modulation by vitamin E. Am J Clin Nutr 58: 98–102.
Brown JE, Wahle KWJ 1990 Effect of fish-oil and vitamin E supplementation on lipid peroxidation and whole-blood aggregation in man. Clin Chim Acta 193: 147–156.
Hoffman DR, Uauy R 1992 Essentiality of dietaryω-3 fatty acids for premature infants: plasma and red blood cell fatty acid composition. Lipids 27: 886–895.
Uauy R, Hoffman DR, Birch EE, Birch DG, Jameson DM, Tyson J 1994 Safety and efficacy of ω-3 fatty acids in the nutrition of very low birth weight infants: soy oil and marine oil supplementation of formula. J Pediatr 124: 612–620.
Lepage G, Roy CC 1986 Direct transesterification of all classes of lipids in one-step reaction. J Lipid Res 27: 114–120.
Onkenhout W, Van der Poel PF, Van den Heuvel MP 1989 Improved determination of very-long-chain fatty acids in plasma and cultured skin fibroblasts: application to the diagnosis of peroxisomal disorders. J Chromatogr 494: 31–41.
Yamamoto Y, Niki E, Eguchi J, Kamiya Y, Shimasaki H 1985 Oxidation of biological membranes and its inhibition: free radical chain oxidation of erythrocyte ghost membranes by oxygen. Biochim Biophys Acta 819: 29–36.
Thurnham DI, Smith E, Flora PS 1988 Concurrent liquid-chromatographic assay of retinol, α-tocopherol, β-carotene,α-carotene, lycopene, and β-cryptoxanthin in plasma with tocopherol acetate as internal standard. Clin Chem 34: 377–381.
Folch J, Less M, Sloam-Stanley CH 1957 A single method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–502.
Speek AJ, Schrijver J, Schreurs WHP 1984 Fluorometric determination of total vitamin C in whole blood by high-performance liquid chromatography with pre-column derivatization. J Chromatogr 305: 53–60.
Asakawa T, Matsushita S 1980 Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 15: 137–140.
Yagi K 1976 A simple fluorimetric assay for lipoperoxide in blood plasma. Biochem Med 15: 212–216.
Das BS, Thurnham DI, Patnaik JA, Das DB, Satpathy R, Bose TK 1990 Increased plasma lipid peroxidation in riboflavin-deficient, malaria-infected children. Am J Clin Nutr 51: 859–863.
Redegeld FAM, Van Opstal MAJ, Houdkamp E, Van Bennekom WP 1988 Determination of glutathione in biological material by flow-injection analysis using an enzymatic recycling reaction. Anal Biochem 174: 489–495.
Stocks J, Dormandy TL 1971 The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol 20: 95–111.
Jain SK 1986 Membrane lipid peroxidation in erythrocytes of the newborn. Clin Chim Acta 161: 301–306.
Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH, Tolley EA 1991 Long-term feeding of formulas high in linolenic acid and marine oil to very low birth weight infants: phospholipid fatty acids. Pediatr Res 30: 404–412.
Van Gossum A, Shariff R, Lemoyne M, Kurian R, Jeejeebhoy K 1988 Increased lipid peroxidation after lipid infusion as measured by breath pentane output. Am J Clin Nutr 48: 1394–1399.
Wispe JR, Bell EF, Roberts RJ 1985 Assessment of lipid peroxidation in newborn infants and rabbits by measurements of expired ethane and pentane: influence of parental lipid infusion. Pediatr Res 19: 374–379.
Pitkänen OM, Hallman M, Andersson SM 1990 Correlation of free oxygen radical-induced lipid peroxidation with outcome in very low birth weight infants. J Pediatr 116: 760–764.
Sosenko IRS, Rodriquez-Pierce M, Bancalari E 1993 Effect of early initiation of intravenous lipid administration on the incidence and severity of chronic lung disease in premature infants. J Pediatr 123: 975–982.
Choi J-H, Yu BP 1990 Unsuitability of TBA test as a lipid peroxidation marker due to prostaglandin synthesis in the ageing kidney. Age 13: 61–64.
Frei B, England L, Ames BN 1989 Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 86: 6377–6381.
Van Zoeren-Grobben D, Lindeman JHN, Houdkamp E, Brand R, Schrijver J, Berger HM 1994 Postnatal changes in plasma chain-breaking antioxidants in healthy preterm infants fed formula and/or human milk. Am J Clin Nutr 60: 900–906.
Van Zoeren-Grobben D, Schrijver J, Van den Berg H, Berger HM 1987 Human milk vitamin content after pasteurisation, storage, or tube feeding. Arch Dis Child 62: 161–165.
Clahsen PC, Moison RMW, Holtzer CAJ, Berger HM 1992 Recycling of glutathione during oxidative stress in erythrocytes of the newborn. Pediatr Res 32: 399–402.
McCoy RN, Hill KE, Ayon MA, Stein JH, Burk RF 1988 Oxidant stress following renal ischemia: changes in the glutathione redox ratio. Kidney Int 33: 812–817.
Németh I, Boda D 1994 Blood glutathione redox ratio as a parameter of oxidative stress in premature infants with IRDS. Free Radic Biol Med 16: 347–353.
Hart CM, Tolson JK, Block ER 1991 Supplemental fatty acids alter lipid peroxidation and oxidant injury in endothelial cells. Am J Physiol 260:L481–L488.
Reaven P, Parthasarathy S, Grasse BJ, Miller E, Almazan F, Mattson FH, Khoo JC, Steinberg D, Witztum JL 1991 Feasibility of using oleate-rich diet to reduce the susceptibility of low-density lipoprotein to oxidative modification in humans. Am J Clin Nutr 54: 701–706.
Acknowledgements
The authors thank Ralf Moison and Adriaan Haasnoot for their help, and the nurses of the Juliana Kinderziekenhuis for their assistance. We also thank the pediatricians Frans Jansen and Paul van Zwieten for their help with follow-up of the patients.
Author information
Authors and Affiliations
Additional information
Supported with a grant from Nutricia N.V., The Netherlands.
Rights and permissions
About this article
Cite this article
Jacobs, N., Van Zoeren-Grobben, D., Drejer, G. et al. Influence of Long Chain Unsaturated Fatty Acids in Formula Feeds on Lipid Peroxidation and Antioxidants in Preterm Infants. Pediatr Res 40, 680–686 (1996). https://doi.org/10.1203/00006450-199611000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199611000-00006