Abstract
Our purpose was to study the effect of dexamethasone (DEX) on choroidal(ChBF) and retinal blood flow (RBF) during normoxia and hyperoxia. Eighteen spontaneously breathing newborn piglets were examined. ChBF and RBF were measured using radiolabeled microspheres while the piglets were in normoxia before (RA1) and 45 min after either saline or DEX (2 mg/kg) infusion(RA2), and after 90 min of hyperoxia (O2) (Pao2 40-60 kPa). Vitreous prostanoids (prostaglandins F1α and E2 and thromboxane B2) and leukotrienes (leukotriene B4) measurements were obtained during normoxia after either placebo or DEX infusion in an additional 22 piglets. Vitreous prostanoids were also studied after 90 min of hyperoxia. We found that RBF increased significantly after DEX infusion(p < 0.02). There was no change in RBF from RA1 to RA2, before and after saline infusion. RBF decreased significantly during hyperoxia in both groups (p < 0.03). ChBF did not change significantly between RA1 and RA2 in any of the groups. ChBF decreased significantly during hyperoxia in both groups (p < 0.03). Vitreous prostanoids and leukotrienes were reduced significantly after DEX infusion (p < 0.05). Prostanoids were similar in the two groups during hyperoxia. We concluded that DEX increases RBF significantly, but not ChBF. RBF and ChBF decreased in both groups during hyperoxia. Therefore, the metabolites of arachidonic acid do not seem to be involved as mediators of hyperoxic vasoconstriction.
Similar content being viewed by others
Main
The fetus and neonate are frequently exposed to DEX for different therapeutic indications. Among others, DEX is administered to the mother when preterm delivery is inevitable. In neonates DEX is being used mainly in the treatment of bronchopulmonary dysplasia, although pretreatment with DEX before extubations has been suggested to be beneficial(1). Furthermore, early use of DEX to prevent the development of bronchopulmonary dysplasia has been tried(2).
DEX has been postulated as a possible risk factor in the development of severe ROP(3, 4). Sobel et al.(5) reported reduced need for cryotherapy in DEX-treated infants. On the other hand, it is not known if DEX has any hemodynamic effects on ocular blood flow. Thus, DEX may alter basal ocular blood flow or modulate the ocular blood flow response to blood gas changes.
DEX has different modes of pharmacologic actions. It is known to be a phospholipase A inhibitor, and hence may modify or inhibit the synthesis of arachidonic acid(6). This would affect the synthesis of both cyclooxygenase and lipoxygenase metabolites.
Hyperoxia is still regarded as the major risk factor in the pathogenesis of ROP(7, 8). However, its mode of action is still unknown. Hyperoxia causes vasoconstriction of both the retinal and choroidal vasculature(9). The importance of this vasoconstriction is by some authors believed to be protective(10, 11) and by others to be pathogenic(12, 13). Whether this vasoconstrictive action of hyperoxia on the blood vessels is mediated by vasoactive substances or by a direct effect on the vessel wall is not yet demonstrated. It has been shown that the prostanoids do not seem to be involved in the vasoconstrictive response to hyperoxia(9). On the other hand, an increased production of lipoxygenase metabolites in vessels exposed to hyperoxia has been reported(14). Hence DEX may modulate the effect of hyperoxia on RBF and ChBF. Thus, the aim of this study was to examine whether DEX modulates resting retinal and choroidal vascular tone and furthermore modifies the response to hyperoxia.
METHODS
Newborn piglets ≤7 d old were anesthetized with ketamine (20 mg/kg intramuscularly) and xylazine (2 mg/kg intramuscularly) for surgical procedures. Lidocaine hydrochloride (0.5%) was used as local anesthesia. After the induction of anesthesia, the animals were sedated with chloral hydrate(100 mg/kg intraperitoneally), which was repeated at 50 mg/kg, every 3 h throughout the study period. Experimental trials were done at least 2 h after the administration of ketamine and xylazine.
The left femoral artery and vein were cannulated and used for ABP and blood gas measurements, and infusion of maintenance fluid and drugs. A 3.5 F catheter (Sherwood Medical, St. Louis, MO) was placed in the left ventricle via the right brachial artery under pressure guidance and used for microsphere injections. The position of the catheter was later verified during the autopsy. A 5 F Swan Ganz catheter was inserted under fluoroscopic guidance via the right external jugular vein into the left pulmonary artery for cardiac output measurements. The left brachial artery was also cannulated and used to withdraw blood at a constant rate to obtain a reference blood sample. Cardiac output was measured by thermodilution using a cardiac output computer(Oximetrix 3 SO2/CO computer, Abbot, Chicago, IL), and ABP was measured with a pressure transducer (DXT Pressure Transducer, Viggo-Spectramed, Oxnard, Ca) and recorded on a multichannel recorder (Cardioswiss CM-8, Schiller Instruments, Switzerland).
Rectal temperature was monitored with a thermistor probe (Yellow Springs Instrument Co., Yellow Spring, OH). The rectal temperature was maintained constant by means of a servo-controlled radiant warmer. The animals received an infusion of 5% dextrose solution (6 mL/kg/h).
This study protocol was approved by the Animal Care Committee of the Autonoma University of Madrid and conducted according to the guidelines of the National Institutes of Health.
Blood flow measurements. Microspheres with a diameter of 15± 1 μm (mean ± SD) labeled with one of the following radionuclides: 51Cr, 57Co, and 81Sr (DuPont NEN, Boston, MA) were used in random order to measure organ blood flow(15). Approximately 1.0-1.2·106 microspheres were injected and flushed with 4 mL of warmed saline into the left ventricle over a period of 20-30 s. Before the injection, the microsphere solution was sonicated and vigorously shaken. A reference blood sample was continuously withdrawn from the brachial artery catheter 10 s before and during, and 60 s after, microsphere injection at a rate of 0.97 mL/min, using a constant flow rate withdrawal pump (Harvard Apparatus, Millis, MA).
After the final blood flow determination, the animal was killed by an Euthane overdose. Radioactivity in retina, choroid, and blood samples was measured in a two-channel gamma-counter (GAMMAtik I, Kontron, Everett, MA). Blood flow to the various tissues was calculated according to the equation(15) Q = At·Q/Ar whereAt and Ar are the activities (counts/min) in the tissue and reference blood, and Q is the rate of withdrawal of the blood sample. Blood flow to each tissue was expressed per 100 g of tissue.
Prostanoid and leukotriene measurements. To test the phospholipase A inhibitor efficiency of DEX in the eye, vitreal samples were obtained for prostanoid and leukotriene measurements. The samples were collected in prechilled polypropylene tubes pretreated with DEX. All samples were centrifuged for 15 min. The supernant was extracted and stored at -45°C until the day of assay. The prostanoids (PGF1α, PGE2, and TXB2) and leukotrienes (leukotriene B4) were measured using RIA as described by others(16).
Experimental protocols. We chose to have separate groups for blood flow studies and biochemical studies because withdrawal of vitreous changes the intraocular pressure which would invalidate the blood flow measurements(17). However, the prostanoid measurements after hyperoxia could be taken from the animals studied for blood flow, because they were withdrawn after the last blood flow measurement.
Group 1. Eighteen newborn piglets were randomly assigned to either a control group (n = 9; weight, 1.85 ± 0.1 kg) or a DEX group (DEX) (n = 9; weight, 1.92 ± 0.1 kg). A stabilization period of 90 min was allowed after the surgery. ABPs, arterial blood gases, and blood flow measurements were obtained in RA as baseline(RA1). Then saline (control) or DEX, 2 mg/kg i.v., was administered. The same measurements were then repeated 45 min after the infusion(RA2). Both measurements were performed during normoxia. The animals were then submitted to hyperoxia (Pao2 = 40-60 kPa) for 90 min by connecting the animals to a breathing circuit with a constant flow of 4-5 L/min with Fio2 of 1.0. At the end of this period the measurements were repeated (O2), while the animals were still breathing 100% oxygen. In 12 animals (six animals in each group) vitreous was taken for prostanoid measurements after the last blood flow measurement.
Group 2. To asses the effect of time on the physiologic measurements (arterial blood gases, pH, cardiac output, blood pressure, and blood flow), five additional animals (weight, 1.60 ± 0.1 kg) were studied in normoxic conditions. The same protocol as above was followed using normoxia in place of hyperoxia.
Group 3. This group (n = 22), underwent the same surgical procedures as group 1. The animals were randomly divided into a control group (n = 11) or a DEX group (n = 11). However, in this group vitreous was taken only in RA 45 min after either saline or DEX infusion (RA2). Due to small sample volume of the vitreous, either prostanoid or leukotriene measurements could be performed. Thus, in the DEX group prostanoids were measured in six animals and leukotrienes in five. In the control group six were used for prostanoids and five for leukotriene measurements.
Hemoglobin concentration and hematocrit levels were obtained at the beginning and end of the experiment.
Statistical analyses. The patterns of blood flow data were analyzed using analysis of variance for repeated measurement. When a significant F value was found, Dunnet's t test was applied for further analyses. The t test was used to analyze the hemodynamic parameters and vascular resistance. Biochemical data were analyzed using nonparametric comparisons of two samples (Wilcoxon). The data are presented as median with upper and lower quartile when nonparametric analyses were used, otherwise as mean ± SEM. A p < 0.05 was considered significant.
RESULTS
Blood flow. Blood gases, cardiac output, and ABP are shown inTable 1. As intended there is a significant but a similar increase in Pao2 in both groups during hyperoxia. Paco2 remained stable throughout the study period and did not differ between groups. In both groups, ABP decreased significantly during hyperoxia (p < 0.04). Regarding cardiac output, there was a small decrease during hyperoxia only in the control group (p < 0.03).
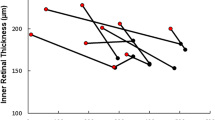
RBF and ChBF are shown in Figs. 1 and2. In the treatment group RBF (Fig. 1) increased significantly after DEX infusion (p < 0.02), whereas there were no differences between RBF at RA1 and RA2 in the control group. In both the DEX group and control group hyperoxia significantly reduced RBF (p < 0.03). Figure 2 depicts ChBF. In neither the DEX group nor the control group were there any significant change from RA1 to RA2. In both groups, ChBF decreased significantly during hyperoxia(p < 0.03).
DEX caused a decrease in retinal vascular resistance from 0.98 ± 0.2 to 0.84 ± 0.2 (p < 0.02), whereas no significant effect on the choroidal vascular resistance was found. Retinal vascular resistance increased from 0.84 ± 02 (after DEX) to 1.51 ± 0.4 mm Hg/mL/min/100 g (80%) (p < 0.02) during the hyperoxic condition. Also choroidal vascular resistance showed a tendency to increase, from 0.02± 0.005 to 0.03 ± 0.004 mm Hg/mL/min/100 g (50%) (p< 0.07). In the control group retinal vascular resistance increased from 0.9 ± 0.1 (after saline) to 1.2 ± 0.1 mm Hg/mL/min/100 g (30%)(p < 0.02) during hyperoxia. The choroidal vascular resistance changed from 0.018 ± 0.002 to 0.023 ± 0.003 mm Hg/mL/min/100 g(28%) (p < 0.06).
RBF, ChBF, arterial blood gases, pH, ABP, and cardiac output were stable throughout the study period in the five animals studied under normoxic conditions (Table 2). This rules out the possibility that the changes observed in the hyperoxic animals were influenced by the length of the experiment.
Prostanoid and leukotrienes results. The prostanoid and the leukotriene results are shown in Table 3. In the piglets that received DEX, vitreous PGF1α, PGE2, TXB2, and leukotriene B4 levels were significantly lower (p < 0.05) than in the piglets that received only saline. Only prostanoid could be studied after hyperoxia due to volume size. In the control group, PGF1α, PGE2, and TXB2 decreased during hyperoxia. In the DEX group there was no change between PGF1α, PGE2, and TXB2 during normoxia and hyperoxia. Furthermore, PGF1α, PGE2, and TXB2 levels during hyperoxia were similar in the two groups (Table 3).
DISCUSSION
In the present study, DEX increased RBF but did not affect ChBF. Furthermore, DEX did not modulate the vascular response to hyperoxia, either in the choroidal or the retinal vessels. This suggests that the metabolites of arachidonic acid are involved in maintaining the normal retinal vascular tone. The decrease in RBF and ChBF during hyperoxia agrees with our earlier reported data(8). Thus, the metabolites of arachidonic acid do not seem to be mediators of the vascular response to hyperoxia.
It has been shown that the cyclooxygenase blocker ibuprofen did not modify basal RBF(9, 18), suggesting that resting RBF was not modulated by cyclooxygenase metabolites of arachidonic acid. Thus, we speculate that the increase in RBF after DEX infusion may be attributed to the decrease in leukotrienes. It has been shown that the lipoxygenase metabolites are mainly vasoconstrictors(19, 20). The present study showed a significant decrease in the vitreal leukotriene level after DEX. Thus, the resting retinal vascular tone may be predominated by the vasoconstrictor effect of the leukotrienes. However, other pharmacologic effects of DEX may also be involved resulting in vasodilated retinal vessels(21–24). Thus, it has been depicted that DEX increases the release of natriuretic peptides(21, 22), stabilizes cell membranes, and inhibits free oxygen radical reactions(23, 24), all properties that may cause vasodilation.
Hyperoxia seems to reduce vitreal prostanoids in the control group. In the DEX group, prostanoid levels did not differ during normoxia and hyperoxia. Thus, if prostanoids were involved, the hyperoxic vasoconstriction of the retinal and choroidal vessels should have been abolished or modulated. There were no differences in the retinal and choroidal vascular resistance between groups during hyperoxia. If the reduced levels of vitreal prostanoids explain the vasoconstriction occurring during hyperoxia, one would also expect DEX to cause vasoconstriction and not vasodilation.
Jackson et al.(25) reported a significant inhibition of hyperoxic vasoconstriction in the hamster pouch after leukotriene antagonist infusion. Therefore, although it seems that in some organs the leukotrienes may be involved in the hyperoxic vasoconstrictive response, other mechanisms may contribute to the retinal and choroidal vasoconstriction. The mechanisms are not yet fully understood and require further investigation. One possible mechanism is that hyperoxia has a direct effect on the retinal and choroidal vessel wall. This could result in changing the membrane potential and influencing Ca2+ influx and subsequently the intra- and extracellular Ca2+. Thus, it has been shown that an alteration in the Na+-Ca2+ exchange system may increase vascular tension(26). However, other mediators such as endothelin, which is a potent vasoconstrictor, could be implicated. Endothelin has been demonstrated in neural tissue(27) and in some ocular structures(28) and thus may also be present in the retina. Chronic hypoxia triggers endothelin release, but whether acute hyperoxia influences endothelin release is not known.
Inasmuch as ABP did not differ between the control and DEX groups, the decrease in cardiac output cannot explain the ABP response to hyperoxia. The mechanisms explaining the reduction of ABP during hyperoxia are not known. It may be a consequence of vasodilation in other vascular beds caused by hyperoxia (data obtained in our laboratory). It is possible that this fall in ABP during hyperoxia may influence the decrease in RBF. However, the RBF is well autoregulated(18, 29). In addition, we have previously shown that the RBF autoregulation was well preserved in newborn piglets with blood pressure as low as 30 mm Hg during normoxia(30). In the present study, only one animal in each group presented with a blood pressure lower than 30 mm Hg (28 and 29 mm Hg). It is therefore unlikely that ABP alone can explain the decrease in RBF observed during hyperoxia. Thus, there was a significant change in the retinal vascular resistance with hyperoxia. Previously we have shown that retinal vascular resistance changed as a function of mean systemic blood pressure to maintain a constant RBF(30). Therefore, we would have expected a decrease in retinal vascular resistance after the decrease in blood pressure during hyperoxia. However, in the present study retinal vascular resistance increased during hyperoxia. The discrepancy observed between the ABP and retinal vascular resistance is probably due to vasoconstriction caused by hyperoxia.
In contrast, the ChBF is not as well regulated and, therefore, the decrease in ABP may in part explain the decrease in ChBF observed during hyperoxia(29, 30). On the other hand, the choroidal vascular resistance showed a tendency to increase during hyperoxia, indicating vasoconstriction (p < 0.06 in the control group and p< 0.07 in the DEX group).
Furthermore, Chemtob et al.(18) demonstrated that ChBF was stable within an ABP range of 17-117 mm Hg after the administration of a cyclooxygenase blocker. Thus, if the decrease in ChBF during hyperoxia was caused by the decrease in ABP, a different response should be expected in the saline- and DEX-treated animals during hyperoxia.
The apparent greater increase in vascular resistance in the DEX group during hyperoxia is probably due to hyperoxia acting on vasodilated vessels. The increase in vascular resistance from the baseline (RA1) to the hyperoxia did not reveal any differences between the groups.
In conclusion, DEX caused an increase in basal RBF, but not in ChBF. Furthermore, the hyperoxic vasoconstriction in both vascular beds was neither inhibited nor modified by DEX. Hence, other mediators or mechanisms than the metabolites of arachidonic acid are involved in the retinal and choroidal vascular response to hyperoxia.
It has been demonstrated that DEX increase ocular blood velocity in the neonate(31). Thus, we speculate that the increase in RBF caused by DEX may have contrary effects on the retina depending on the actual oxygen saturation of the infant. In the newborn kitten Phelps and Rosenbaum(32) and Phelps(33) demonstrated that chronic hypoxemia during ROP healing adversely affects ROP. Recently, Penn et al.(34) showed that hypoxia plays a role in the pathogenesis of ROP. Thus, if an increase in RBF improves retinal oxygenation in a baby with marginal hypoxemia, DEX may be beneficial. On the other hand, in a well oxygenated baby, DEX may be harmful, because an increase in RBF may result in augmented oxygen delivery to the retina. This may provoke oxygen tissue toxicity. It may well be that differences in babies' oxygen saturation explain the fact that in some studies DEX was beneficial(5) and in others DEX had adverse effects on ROP(3, 4). However, the other pharmacologic properties of DEX may also play a part.
Abbreviations
- RBF:
-
retinal blood flow
- ChBF:
-
choroidal blood flow
- ABP:
-
arterial blood pressure
- RA:
-
room air
- ROP:
-
retinopathy of prematurity
- DEX:
-
dexamethasone
- PG:
-
prostaglandin
- TXB2:
-
thromboxane B2
- Fio2:
-
fractional concentration of inspired O2
References
Couser RJ, Ferrara B, Falde B, Johnson K, Schilling CG, Hoekstra RE 1992 Effectiveness of dexamethasone in preventing extubation failure in preterm infants at increased risk for airway edema. J Pediatr 121: 591–596
Yeh TF, Torre JA, Rastogi A, Anyebuno MA, Pildes RS 1990 Early post-natal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double-blind controlled study. J Pediatr 117: 273–282
Batton DG, Roberts, Trese M, Maisels MJ 1992 Severe retinopathy of prematurity and steroid exposure. Pediatrics 90: 534–536
Asztalos EV, Zayack D, Shennan AT, Ohlsson A, Girschek P 1993 Dexamethasone-a risk factor for aggressive retinopathy of prematurity. Pediatr Res 29: 200A
Sobel DB, Philips AGS 1992 Prolonged dexamethasone therapy reduces the incidence of cryotherapy for retinopathy of prematurity in infants of less than 1 kilogram birth weight with bronchopulmonary dysplasia. Pediatrics 90: 529–532
Coyne DW, Nickols M, Bertrand W, Morrison AR 1992 Regulation of mesangial cell cyclooxygenase synthesis by cytokines and glucocorticoids. Am J Physiol 263:F97–F102
Lucey JF, Dangman B 1984 A reexamination of the role of oxygen in retrolental fibroplasia. Pediatrics 73: 82–96
Silverman WA 1988 The oxygen hypothesis: fruitful predictor or narrow dogma. Birth Defects Orig Art Ser 24: 203–207
Stiris TA, Suguihara C, Flynn J, Quero J, Bancalari E 1996 Effects of the cyclooxygenase inhibitor ibuprofen on retinal and choroidal blood flow during hyperoxia in newborn piglets. Biol Neonate 69: 101–108
Flower RW, McLeod D, Wajer SD, Sendi GS, Egner PG, Dubin NH 1984 Prostaglandins as mediators of vasotonia in the immature retina. Pediatrics 73: 440–444
Kretzer LK, Hittner HM 1988 Spindle cells and retinopathy of prematurity: Interpretations and predictions. Birth Defects Orig Art Ser 24: 147–168
Patz A 1968 The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc 66: 940–985
Ashton N, Cook C 1954 Direct observation of the effect of oxygen on the developing vessels: preliminary report. Br J Ophthalmol 38: 433–440
Stuart MJ, Walenga RW, Setty BNY, Phelps DL 1990 Effects of changes in oxygen tension on lipoxygenase metabolites. Biol Neonate 57: 313–317
Heymann MA, Bruce DP, Hoffman JIE, Rudolph AM 1977 Blood flow measurements with radionuclide-labelled particles. Progr Cardiovasc Dis 20: 55–78
Mahlberg K, Uustitalo R, Palkama A, Tallberg T 1987 Phospholipase A2, leukotriene C4 and prostaglandin E2 levels in aqueous humor of guinea pigs with experimental S-antigen induced autoimmune uveitis. Curr Eye Res 6: 321–335
Bill A 1962 Intraocular pressure and blood flow through the uvea. Arch Ophthalmol 67: 90–102
Chemtob S, Beharry K, Rex J, Chatterjee T, Varma DR, Aranda JV 1991 Ibuprofen enhances retinal and choroidal blood flow autoregulation in newborn piglets. Invest Ophthalmol Vis Sci 32: 1799–1807
Uski TK, Hogestatt 1992 Effect of various cyclooxygenase and lipoxygenase metabolites on guinea-pig cerebral arteries. Gen Pharmacol 21: 109–113
Stjernschantz J, Sherk T, Sears ML 1986 Ocular responses to leukotriene C4 and D4 in the cat. Prostaglandins 27: 5–16
Gardner DG, Hane S, Trachewsky D, Schenk D, Baxter JD 1986 Arterial natriuretic peptide mRNA is regulated by glucocorticoidsin vivo. Biochem Biophys Res Commun 139: 1047–1054
Danaberg J, Grekin RJ 1992 Corticoid regulation of atrial natriuretic factor secretion and gene expression. Am J Physiol 263:H1377–H1381
Demopoulos HB, Flamm ES, Pietronigro DD 1980 The free radical pathology and microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl 492: 91–119
Bangham AD, Standish MM, Weissmann G 1965 The action of steroids and streptolysin S on the permeability of phospholipid structures to cations. J Mol Biol 13: 253–259
Jackson WF 1989 Arteriolar oxygen reactivity is inhibited by leukotriene antagonist. Am J Physiol 257:H1565–H1572
Evangeline MD, Richard PJ, Matlib MA 1993 Role of Na+-Ca2+ exchange in the regulation of vascular smooth muscle tension. Am J Physiol H1028–H1040
Greenberg DA, Chan J, Sampson HA 1992 Endothelins and the nervous system. Neurology 42: 25–31
MacCumber MW, Jampel HD, Snyder SH 1991 Ocular effects of endothelins. Abundant peptides in the eye. Arch Ophthalmol 109: 705–709
Bill A 1984 Circulation in the eye. In: Robert B, Sperelakis N (eds) Handbook of Physiology. Williams & Wilkins, Baltimore, pp 1001–1034
Odden JP, Bratlid D, Hall C, Farstad T, Stiris TA 1993 The effect of hypoxemia and hypovolemia on retinal and choroidal blood flow in the newborn piglet. Biol Neonate 64: 140–150
Pellicer A, Gaya F, Valverde E, Stiris T, Quero J, Cabanas F 1995 Effect of dexamethasone (DEX) therapy on cerebral haemodynamics studied by colour doppler flow imaging and near-infrared spectroscopy (NIRS). Pediatr Res 38: 449A
Phelps DL, Rosenbaum AL 1984 Effects of marginal hypoxemia on recovery from oxygen-induced retinopathy in the kitten model. Pediatrics 73: 1–6
Phelps DL 1988 Reduced severity of oxygen-induced retinopathy in kittens recovered in 28% oxygen. Pediatr Res 24: 106–109
Penn JS, Henry MM, Tolman BL 1994 Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 36: 724–731
Author information
Authors and Affiliations
Additional information
Supported by the Unger-Vetlesen's Medical Foundation, the Spanish Department for Education and Science (DIGCYT), and Foundation for Investigation, the Spanish Department of Health (FIS). T.A.S. is also grateful for contributions from The Norwegian Association for the Blind.
Rights and permissions
About this article
Cite this article
Stiris, T., Blanco, D., Codoceo, R. et al. Effects of Dexamethasone on Retinal and Choroidal Blood Flow during Normoxia and Hyperoxia in Newborn Piglets. Pediatr Res 40, 592–596 (1996). https://doi.org/10.1203/00006450-199610000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199610000-00013
This article is cited by
-
Effect of perinatal glucocorticoids on vascular health and disease
Pediatric Research (2017)