Abstract
Effects of light-exposed parenteral solutions on hepatic function and lidocaine metabolism were studied. Male rats (190-220 g) were randomly assigned to one of the three isocaloric treatment groups: parenteral nutrition(PN)(+L) group (n = 6), animals received a solution of 24.2% dextrose and 5.2% amino acids with light exposure; PN(-L) group (n = 5), animals received the same solution without light exposure; and CF group, chow-fed animals (n = 6) that received rat chow and water. Average energy intake in these animals was ≈33 kcal/100 g/d. Lidocaine metabolism was studied in livers isolated from animals 7 d after treatment. Liver morphology indicated that five livers from PN(+L) animals developed steatosis. The lidocaine metabolism rate was the slowest in PN(+L) animals; this is marked by changes in the steady state levels (% dose) of lidocaine [PN(+L), 47.9 ± 17.6; PN(-L), 25.9 ± 2.1; versus CF, 11.3± 5.3; p < 0.05]. The extraction ratio and intrinsic clearance values were reduced by 41 and 86% in PN(+L) group and 16 and 70% in PN(-L) group, respectively, when compared with CF (p < 0.05). Metabolite to drug ratios indicated that N-dealkylation,ring-hydroxylation, and aryl methyl hydroxylation of lidocaine were severely impaired, particularly in the PN(+L) animals. The extents of reduction in metabolic pathways were in the ranges 79-95% in the PN(+L) group and 44-90% in the PN(-L) (p < 0.05). These findings suggest that parenteral nutrition infusion induces steatosis and reduces the rate of lidocaine metabolism and exposure of parenteral nutrition solutions to light exacerbates this change.
Similar content being viewed by others
Main
PN-associated hepatobiliary complications in adults and children have been well established(1, 2). The exact mechanism by which PN induces hepatic dysfunction is not well defined. Factors postulated to be associated with this complication include the formation of photo-decomposed products of amino acids(3–5), excessive amino acid and sugar overload(6, 7), essential fatty acid deficiency(8), and sepsis(9–11). A link between the formation of photooxidized amino acids and PN-associated hepatotoxicity has received much attention lately. Results from a number of studies(4, 5, 12–14) have indicated that solutions of certain amino acids, especially tryptophan, in the presence of light and multivitamins, were toxic to animal liver and brain tissues. Currently most amino acids are marketed in plastic bags protected with aluminum foil, or clear bottles with a label warning to protect from light until use. Unfortunately, these PN solutions, containing amino acids, dextrose, lipid, MVI, and other additives, are often infused from transparent bags and clear tubings without protection from light. Light exposure is particularly prevalent in intensive care nurseries where infants undergo phototherapy for jaundice and so in this environment solutions of amino acids are exposed to intense light. PN infusion solutions in ambulatory patients may even be subjected to direct sunlight. Exposure to these light sources can promote photo-decomposition of amino acids and consequently can induce hepatic dysfunctions and reduced CYP activity.
Furthermore, such infants or adults often have to undergo drug therapy during PN infusion, and how these PN solutions might interact with drugs has not been investigated. The study now reported was designed: 1) to investigate the effect of light exposure of PN solutions on hepatic function and 2) to study the effect of light-exposed PN solutions on the elimination of a model compound, lidocaine, in an animal model.
METHODS
Eighteen male Sprague-Dawley rats weighing 200-230 g were supplied by Biosciences Animal Services, University of Alberta. The protocol of the proposed study met the guidelines of the Canadian Council on Animal Care and the use of animals was approved by the Health Sciences Animal Welfare Committee at the University of Alberta. Each rat was anesthetized with methoxyflurane, and its right jugular vein was catheterized with silastic tubing (outside diameter, 0.047 inch, and inside diameter, 0.025 inch; Dow Corning Corp., Midland MI). The catheter was then tunneled s.c. and exteriorized in the scapular region where it was connected to a coiled metallic spring mounted on a swivel (Rodent Single Channel Swivel, Alice King Chatham Medical Arts, Los Angeles, CA). This connection permitted free mobility of the subject. Surgical equipment was sterilized before each surgery, and the entire surgical procedure was carried out aseptically in a laminar flow hood. After each surgery, the animal was weighed and placed in a metabolic cage that was housed in a well ventilated room where a 12-h light and dark cycle was maintained. Rats were allowed to recover for at least 2 d after surgery, and during this period they had free access to a standard laboratory rodent chow (Richmond Standard, PMI Feed Inc., St. Louis, MO) and water. On d 3, the weights of the animals were recorded.
Animals were randomly assigned to one of three treatment groups: PN(+L), PN solutions of dextrose and amino acids were exposed to light: PN(-L), same treatment as in PN(+L) but PN solutions were protected from light; and CF animals. The PN solution infused into PN(+L) animals was placed approximately 18 cm below a bank of fluorescent lamps for 24 h. The light exposure was maintained at 6 μW/cm2/nm (425-475 nm), identical to that reported by Bhatia et al.(12). This procedure simulates the light intensities of infant intensive care units. Under these conditions, infusate solutions turned brown after a 24-h exposure. Infusion bags were then protected from light by covering them with aluminum foil during the infusion period to avoid further decomposition. Samples were withdrawn before and after light exposure to measure the extent of decomposition. Animals were infused for 7 d at a rate of 3 mL/h. CF rats received i.v. saline at the same rate, delivered by a volumetric infusion pump (model 927, IMED Corp., San Diego, CA), and had free access to rat chow and water. The composition of the PN solution was identical to that reported by Ke et al.(15). Each liter of PN contained 242 g of dextrose, 52 g of amino acids (10% Travasol blend B with electrolytes, Baxter-Travenol Lab., Malton, Canada), 2 mL of MVI (MVI 1000, Aqueous Multivitamin IV infusion, USV Canada Inc.), and 2.25 mmol of calcium gluconate(10% calcium gluconate injection, Squibb Canada Inc., Montreal, Canada). Solutions were prepared aseptically in a laminar flow hood, and containers were immediately covered with aluminum foil to avoid exposure to light. During PN infusion animals were allowed free access only to water. On the 8th d each rat was weighed, and its liver was isolated.
Isolated rat liver perfusion. A “one-pass” liver perfusion method was used in this study. Details of the isolated liver perfusion procedure were reported previously(16–18). Concisely, the portal vein was cannulated with an intravascular “over-the-needle” 16 G Teflon catheter (Quick-cath, Baxter Healthcare Co., Deerfield, IL), which was used to provide oxygenated Kreb's bicarbonate buffer. The effluent perfusate sample was collected from a catheter located in the thoracic inferior vena cava. Liver function was monitored by measuring oxygen consumption, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels in the effluent perfusate at 0 and 70 min, by recording hydrostatic pressure, and by the organ's general physical appearance. The rate of oxygen consumption was 2-3 mL/h/g and was within the reported normal range(19). Aspartate and alanine transferase and alkaline phosphatase values and hydrostatic pressure were constant throughout the experiment. It has been reported that under normal conditions perfused liver will remain viable for at least 3 to 4 h after its removal from the animal(20). Our experiment was completed within 90 min. The viability of the liver was further confirmed by the stable concentrations of lidocaine and its metabolites at steady state during the infusion period. Each liver was infused with lidocaine (3.5 μg/mL at a rate of 30 mL/min) via a Vario pump (Cole Parmer Instrument Co., Chicago IL) for 70 min. This period was found to be sufficient for lidocaine and its metabolites to approach a steady state(16). The inlet concentration of lidocaine(Cin) was determined by obtaining six samples (2 mL each) directly from the inlet reservoir at 0, 12, 22, 33, 44, and 70 min after the initiation of lidocaine infusion. The outlet concentration of lidocaine and its metabolites (Cout) were measured in the effluent samples at 0, 1, 3, 5, 7, 10 min and at 5 min intervals up to 50 min and then at a 10 min interval up to 70 min. After each experiment the liver was blotted dry and its weight was recorded.
Histologic examination. A portion of each liver was stored in 10% formalin before being stained with hematoxylin-eosin and trichrome stain for connective tissue evaluation, and samples were evaluated by Perl's reaction for iron. Histologic examination was performed in a blinded fashion. The degree of steatosis was rated as none, mild, moderate, or severe.
Serum enzymes and amino acid assays. Blood (≈1.5 mL) was collected from the posterior vena cava of all the animals immediately after catheterizing the portal vein, and the serum was separated by centrifugation. Serum values of liver function test values, aspartate and alanine transferase, alkaline phosphtatase, γ-glutamyl transpeptidase, total bilirubin, white blood cell count, and Hb were determined using a Dri-Stat diagnostic kit(Beckman Instrs., Inc., Fullerton, CA) and a Multistat 3 Micro Centrifugal Analyzer. The coefficient of variation of each assay was within ±3%. Individual serum amino acid concentrations were quantified on a Varian 5000 HPLC coupled to a Varian Fluorichrom detector(21). The reproducibility of the assay was within an average deviation of ±2%.
Photooxidation of amino acids. The amino acid concentrations of each PN solution used to infuse animals in PN(+L) group were determined before and after solutions were exposed to the light source. Individual solutions of four amino acids, tryptophan, methionine, histidine, and tyrosine, previously reported to be photosensitive(22–26), were evaluated individually and in conjunction with three common photosensitizers present in a PN solution, namely RB, MVI, and SB, using the same illuminating technique as described above. The initial concentrations of the four amino acids were identical to those found in the PN solution. Solutions were prepared in glass beakers. An aliquot of each solution was first analyzed to confirm the exact initial concentration of each amino acid before light exposure for 24 h while the top of the beaker was covered with Parafilm. The concentrations of RB, 0.2 mg/dL, MVI, 0.2 mL/dL, and SB, 0.14 g/dL, were the same as present in the PN solution. Because the antioxidant SB is present in all amino acid mixtures, we preferred to add SB to each prepared solution containing the individual amino acid with RB or MVI. The effect of SB on each amino acid solution and in the absence of RB and MVI was determined to see whether amino acid decomposition occurred upon light exposure. The amino acids solutions were analyzed by the same HPLC technique that was used for the serum samples. An aliquot of each solution was also analyzed by UV spectrophotometry before and after light exposure at 200-600 nm to determine color changes.
Chemicals and reagents. Samples of lidocaine hydrochloride and four of its metabolites, MEGX, GX, 3-OH-lidocaine, 3-OH-MEGX, and the internal standard EMGX were gifts from Astra Pharmaceuticals (Mississauga, Canada). The remaining two metabolites, MeOH-lidocaine and MeOH-MEGX, were synthesized in our laboratory based on the method of Nelson et al.(27). Their chemical structures and purity were confirmed using NMR, elemental analysis and GC/MS. HPLC grade solvents and reagents were obtained from BDH Inc., Toronto, Ontario.
HPLC assay . Apparatus. The HPLC system consisted of two M-45 pumps, a model 441 UV detector (set at 214 nm), a model 840 data processing station (Waters Associate, Mississauga, Ontario) and a Shimadzu automatic sampler (model SIL-9A Shimadzu Corp., Kyoto, Japan). Separation of lidocaine and its six metabolites was achieved on a C18 LiChrospher 60 RP select B column (5 μm, 125 mm × 4 mm, Chromatography Merck, Darmstadt, F.R., Germany). An HPLC method developed in our laboratory(28) was used to separate and quantify lidocaine and its metabolites.
Standard curves. Standard solutions were prepared by spiking 1-mL volumes of the blank perfusate with various concentrations (0.02-3.5 μg/mL) of lidocaine and its six metabolites. Calibration curves, constructed by plotting the peak area ratio of lidocaine and each of its metabolites to that of the internal standard versus the respective concentration of the drug or metabolite, were linear over the range studied (r > 0.99). Quality control samples, prepared by noninvolved personnel in the laboratory, were used to validate the assay. The quantifiable limit of this assay was 0.02 μg/mL for lidocaine and its six metabolites when a 1-mL aliquot of perfusate sample was used. The percent deviation from expected values as a measure of accuracy was less than 15% for the standards and less than 10% for the quality control samples. The intraday and interday coefficients of variation for standard and quality control samples of lidocaine and its metabolites were within 10%. A stability study revealed that stock solutions of lidocaine and its metabolites were stable for 8 wk when stored at -20°C.
Pharmacokinetic analysis. The times for lidocaine and its metabolites to reach steady state (Tss) were statistically determined according to the method reported(29). The efficiency of the liver in removing lidocaine at steady state was expressed by the following equations: where Q is the buffer perfusion rate, Cin is the inlet drug concentration,Cout is the effluent drug concentration, EH is the drug extraction ratio and Clint is hepatic intrinsic clearance.


Statistical analysis. The Shapiro-Wilks test and the Kolmogorov-Smirnov Goodness of Fit test were first used to test for normality of the data(30). When it was found that data were not normally distributed, Kruskal-Wallis one-way analysis of variance was used to evaluate the difference(31). When a difference was detected, Duncan's multiple comparison test was used to evaluate the differences among the groups. Significance was set at p = 0.05 level. Values are expressed as means ± SD. The SPSS for Windows computer program was used for these statistical calculations(30).
RESULTS
Body and liver weight changes. All animals had similar body weights [PN(+L), 220.58 ± 13.29 g; PN(-L), 213.80 ± 19.59 g; CF, 224.50 ± 22.06 g) before initiation of the experiment. Despite a healthy appearance of all animals at the end of each experiment, only CF animals exhibited a significant weight gain of 7.55 ± 1.52 g/d. In contrast, animals in the PN(+L) and PN(-L) groups did not show any changes in body weight throughout the study. Liver weights were significantly lower in PN(-L) animals only when compared with the other two groups(Table 1). When liver weights were expressed as percent of body weights, PN(+L) animals were found to have the highest value [(PN(+L), 4.72 ± 0.58%; versus PN(-L), 3.69 ± 0.31%; and CF, 3.98 ± 0.30%; p < 0.05].
Liver functions and serum amino acids. There were no significant differences among the groups in any of the LFT values, despite a lower total bilirubin value in the PN(+L) group (Table 2). The white blood cell value was found to be significantly higher in the PN(-L) group, but there was no indication of sepsis as the white blood cell, and Hb values among groups were within the normal ranges reported for rats(5-13 × 103/mm3)(32).
Serum concentrations of glycine, serine, taurine, tryptophan, methionine, phenylalanine, and histidine in both PN groups were significantly higher than CF values (Table 3) (p < 0.05).
Photooxidation of amino acids. The color of the mixed PN solution changed from yellow to brown after light exposure. This was attributed to the Millard reaction(4) in which dextrose and amino acid solutions turn brown on heating. The light source used in this experiment generated heat, and the infusion bags felt warm after the 24-h light exposure. This might be a sufficient explanation of the browning of the solution. In contrast, the solution of all four amino acids changed from yellow to almost colorless in the presence of RB and MVI after 24 h of light exposure. When no additives were present, only tryptophan solutions changed from colorless to yellow after light exposure.
The extent of amino acid decomposition was studied in the PN mixtures and in solutions containing single amino acids. Only one amino acid, methionine, was significantly reduced by 8.3% to 20% in all test conditions except when no sensitizer was present (Table 4). The percent decomposition of methionine in presence of SB alone was not significantly different from the observed changes in the presence of RB or MVI(Table 4). Among the four amino acids, tryptophan showed a small but consistent 5-6% reduction in all of the six test conditions(Table 4). Histidine showed a significant decomposition in the presence of RB. Tyrosine concentration remained unaffected in all tests performed.
From this study, it is apparent that components present in MVI other than RB do not induce changes in the rates of amino acid decomposition. Interestingly, histidine seemed to have been stabilized when present in the PN solutions or in presence of MVI (Table 4). The UV spectrum of tryptophan after light exposure of solution (with or without sensitizers) revealed the presence of a new small absorbance peak at 324 nm, which did not appear to affect the magnitude of the absorbance peak of tryptophan at 298 nm. The presence of an adduct which absorbed at 324 nm may explain the small (5%) decomposition of tryptophan taking place after light exposure in each of the test conditions. Significant alterations to the characteristic UV absorption band of RB in the 300-450 nm range were observed in all four individual amino acid solutions analyzed after light exposure. This change is consistent with the formation of amino acid-RB adducts as previously reported(3, 4, 33, 34), and may be responsible for the loss of the yellow color of the RB- or MVI-containing amino acid solutions after light exposure.
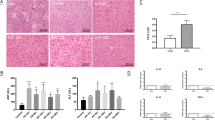
Liver histology. Steatosis was visually characterized by fatty degeneration of hepatocytes and the presence of numerous intracytoplasmic fat globules. Examination of liver specimens from PN(+L) rats revealed that significant steatosis had developed in five out of six liver specimens. Two livers in the PN(+L) animals developed severe steatosis and in the remainder steatosis was moderate. Severe steatosis was evident in the hepatocytes throughout the liver (Fig. 1). Two out of five liver specimens obtained from PN(-L) animals exhibited mild steatosis and contained a few fat droplets (Fig. 2), but there was no evidence of cell injury or inflammation in any of the animals. Liver specimens from CF animals showed a normal cellular architecture (Fig. 3).
Lidocaine elimination. Representative concentrationversus time profiles of lidocaine and its metabolites in the three treatment groups are shown in Figure 4. The effluent lidocaine concentration was the highest in PN(+L) group, and lowest in CF animals. Values are also significantly different in all three groups(Table 1).
A representative concentration vs time profiles of lidocaine and its metabolites in the effluent of perfused rat livers: (a) CF, Cin = 16.21 μM: (b)(amino acids and dextrose protected from light = PN(-L), Cin= 14.56 μM: (c) (amino acids, dextrose exposed to light = PN(+L),Cin = 15.51 μM. •, Lidocaine; ▪, MEGX; ○, GX; □, MeOH-lidocaine; ♦, 3-OH-lidocaine; ▵, MeOH-MEGX; ▴, 3-OH-MEGX.
A comparison of the kinetic parameters provided in Table 1 reveals that the extraction ratio (EH) and intrinsic clearance (Clint) values were lowest in PN(+L) animals(p < 0.05). The mean value of the Clint in the PN(+L) group was approximately one-seventh of that of CF animals, and a reduction of 41% in EH value was observed when PN(+L) animal values were compared with those in CF. These data clearly indicate that lidocaine metabolism was severely compromised, particularly in PN(+L) rats, when PN was exposed to light before infusion.
Steady state percent recoveries of lidocaine and its metabolites are shown in Table 5. A significant increase in the recovery of unchanged lidocaine was observed in both PN groups relative to CF values. MEGX levels were the lowest in the PN(+L) group although a significance was not achieved due to a large variability in MEGX data of PN(+L) group. Levels of MEOH-lidocaine were significantly lower in both PN(+L) and PN(-L) groups. Two metabolites, 3-OH-MEGX and MEOH-MEGX, were not detected in the PN(+L) group and MEOH-MEGX was not detected in the PN(-L) group. Levels of 3-OH-MEGX were similar in the PN(-L) and CF group (Table 6). When ratios of metabolites to lidocaine were compared (Table 6), significant reductions in N-dealkylation, m-hydroxylation, and aryl methyl hydroxylation were observed. Consistent with the kinetic parameters obtained for lidocaine, PN(+L) animals had the most pronounced reduction in lidocaine metabolism.
DISCUSSION
The present study demonstrates for the first time that photooxidized amino acids not only cause hepatosteatosis but also affect hepatic metabolic enzyme activity. Our data show that lidocaine metabolism was impaired in both PN-treated groups but the most dramatic reduction was observed in the PN(+L) animals which displayed significantly reduced enzymatic activities.Clint values were lowered by almost 86% in the PN(+L) group and by 70% in the PN(-L) group, when compared with CF values (p < 0.05). The reduced metabolic activity led to a much higher recovery of unchanged lidocaine particularly in the PN(+L) group. N-Dealkylated,ring-hydroxylated, and aryl methyl hydroxylated metabolic pathways of lidocaine were most affected in the PN(+L) group.Ring-hydroxylation of lidocaine to 3-OH-lidocaine andN-dealkylation to MEGX are major pathways that contribute significantly to the mass balance of lidocaine metabolism. MEGX formation is catalyzed by CYP2C11 and CYP2B1 in rats(35, 36) and by CYP3A4 in humans(37).Ring-hydroxylation in rats is mediated by CYP1A2 and CYP2D enzymes(35, 36, 38), and CYP2B2 is involved in the formation of MEOH-lidocaine(35, 36). All of these enzymes, particularly the ones belonging to the CYP2D subfamily and CYP3A4, are involved in the metabolism of a wide variety of commonly used drugs(39–41). These observations imply that drug treatment during PN infusion should be carefully monitored.
A reduction in the rate of drug metabolism in PN-treated animals have been observed previously(15, 42–45). Our findings in the PN(-L) animals were consistent with that observed by others(15). Therefore, the pronounced reduction of lidocaine elimination in the PN(+L) group may be attributable to the presence of toxic photooxidized amino acid products in this group. Our data suggest that more than one mechanism may be involved in PN-induced metabolic reduction. Some postulated mechanism(s) described in the literature are: 1) amino acid overload/imbalance(3, 15, 44); 2) sugar load(6, 46–48); and 3) sepsis(11, 49).
Some studies have shown a correlation between the ratio of aromatic amino acids to branched chain amino acids and the severity of hepatic encephalopathy. When the ratio of branched chain/aromatic amino acids drops to 1, hepatic encephalopathy prevails, and hepatic encephalopathy improves when the ratio is close to 3(50, 51). In the present study, we found the branched chain/aromatic amino acids ratio to be significantly lower in the PN(+L) and PN(-L) groups compared with CF [PN(+L), 2.53 ± 0.6; PN(-L), 2.1 ± 0.5; versus CF, 2.91± 0.3). This amino acid imbalance could be partly responsible for the observed hepatic abnormalities in the two PN groups, but it is premature to draw any conclusion at this point.
Sepsis and bacterial infection due to an indwelling catheter have been shown to be associated with PN-induced hepatotoxicity(10, 11), which could contribute to a reduction in hepatic drug metabolism(49). However, our CF animals with indwelling catheters had similar rate of lidocaine metabolism when compared with those animals that did not receive any surgery(28). Furthermore, none of our PN-treated or CF animals showed any evidence of sepsis, because all had normal white blood cell and LFT values (Table 3). In addition, our observed serum amino acid levels were not compatible with sepsis(52).
When a sugar load is given in association with a PN solution, a reduction in drug metabolism is observed(47, 53). This has been attributed to an increase in the rate of lipogenesis, a process that would compete with CYP enzymes for NADPH(47, 48) and be partly responsible for the observed hepatotoxic effect observed in our study.
Liver histologic examinations showed that severe steatosis was present in livers of all of the animals that received a PN solution and were exposed to light (Fig. 1). Bhatia et al.(54) reported a depletion of biliary glutathione in animals which developed hepatotoxic effects after having received PN solutions that had been exposed to light. Glutathione is essential for the detoxification of a number of xenobiotics. We did not measure levels of glutathione but it is possible that its depletion partly contributed to the observed hepatotoxicity in our PN(+L) animals.
Research so far has been focused mainly on tryptophan, and studies have revealed that when tryptophan was administered as a single amino acid solution or in a nutrition solution which contained RB, the conversion products formed were toxic to both human and rat livers(3, 4, 34). These observations are particularly important because of the widely reported incidence of liver dysfunction in neonates and in adults that could result from the presence of photooxidized amino acid products.
Although toxicity induced by tryptophan decomposition products have been well documented(3–5, 23, 33, 34). However, the decomposition of tryptophan is minimal in this study(5-6% in all six test conditions; Table 4), and yet a severe liver toxicity was observed. This raises a doubt about whether tryptophan plays any role in the photooxidized PN-induced hepatotoxicity. The discrepancy between our data and those reported by others may be due to various factors: a) the extent of photooxidation is dependent upon the dose of tryptophan(4, 5), quantity of sensitizer present(23), and the duration of light exposure(4, 33). In all of these previous studies the concentrations of tryptophan and the sensitizers were severalfold higher, and the duration of light exposure was longer than what was used in our study. We conclude that drastic hepatic damage in our PN(+L) animals cannot be attributed to the minor decomposition of tryptophan that occurred.
Solutions of various amino acids, particularly tryptophan, methionine, tyrosine, cysteine, and histidine, have been reported to be highly photosensitive(22–26). Light exposure of solutions, especially those that also contain vitamins, particularly RB, accelerates the photooxidation of these amino acids(13, 24–26, 55). Our results clearly indicate that the extent of methionine decomposition was the highest when sensitizers were present (Table 4). Because the quantities of the sensitizers used were same as with the other three amino acids, the methionine-RB adduct that formed would account approximately for only 0.02% of total methionine. The larger (13%) decomposition of methionine clearly indicates that other products besides an adduct had formed. Studies(55, 56) have shown that photooxidation of methionine yields methionine sulfoxide which is hepatotoxic(57–59). Therefore, the possibility that decomposed methionine product(s) induced the hepatotoxicity observed in the present study should not be overlooked. Further studies should be conducted to isolate and identify decomposition products of methionine. It is important to mention at this point that solutions of amino acids that also contained the same sensitizers did not undergo any amino acid decomposition during a period of 10 d when protected from light.
Despite a small reduction in levels of some amino acids after light exposure in the PN(+L) group, the serum levels of most amino acids were higher in the light-exposed PN group (Table 3). This elevation is presumably a secondary effect of the toxic amino acid products on the liver that perturbed the efficiency by which the amino acids would ultimately get used in vivo(60). Significant elevations of certain serum amino acids during PN infusion with or without light exposure is very common(12, 15).
In conclusion, our results indicate the following: 1) PN infusion after light exposure caused severe hepatic damage and a reduction in lidocaine metabolism in rats, and 2) of all of the amino acids investigated, methionine underwent greatest decomposition after light exposure. It is possible that the significant steatosis observed in rats and the pronounced reduction in lidocaine metabolism that occurred could be attributed to methionine toxicity.
These results suggest that PN solutions with or without vitamins should always be protected from light during infusion to minimize hepatotoxicity in human subjects. It may even be a good practice to add the vitamin just before infusion.
Abbreviations
- PN:
-
parenteral nutrition
- PN(-L):
-
light-protected PN group
- PN(+L):
-
light-exposed PN group
- CF:
-
chow-fed
- Cl int :
-
intrinsic clearance
- C in :
-
inlet concentration of lidocaine
- C out :
-
outlet concentration
- CYP:
-
cytochrome P450
- E H :
-
extraction ratio
- EMGX:
-
2-(N-ethyl-N-methylamino)-N-(2,6-dimethylphenyl)acetamide
- GX:
-
2-amino-N-(2, 6-dimethylphenyl)acetamide
- MVI:
-
multivitamin
- MEGX:
-
2-(ethylamino)-N-(2,6-dimethylphenyl)acetamide
- MeOH-MEGX:
-
2-(ethylamino)-N-(2-hydroxymethyl-6-methylphenyl)acetamide
- MeOH-lidocaine:
-
2-(diethylamino)-N-(2-hydroxymethyl-6-methylphenyl)acetamide
- 3-OH-lidocaine:
-
2-(diethylamino)-N-(2,6-dimethyl-3-hydroxyphenyl)acetamide
- 3-OH-MEGX:
-
2-(ethylamino)-N-(2,6-dimethyl-3-hydroxyphenyl)acetamide
- Q :
-
buffer perfusion rate
- RB:
-
riboflavin
- SB:
-
sodium bisulfite
References
Fisher RL 1989 Hepatobiliary abnormalities associated with total parenteral nutrition. Gastroenterol Clin N Am 18: 645–666
Balistreri WF, Bove KE 1990 Hepatobiliary consequences of parenteral alimentation. Prog Liver Dis 9: 567–601
Grant JP, Cox CE, Kleinman LM 1977 Serum hepatic enzyme and bilirubin elevations during parenteral nutrition. Surg Gynecol Obstet 145: 573–580
Merritt RJ, Sinatra FR, Henton D, Neustein H 1984 Cholestatic effect of intraperitoneal administration of tryptophan to suckling rat pups. Pediatr Res 18: 904–907
Bhatia J, Rassin DK 1985 Photosensitized oxidation of tryptophan and hepatic dysfunction in neonatal gerbils. JPEN 9: 491–495
Buzby GP, Mullen JL, Stein TP, Rosato EF 1981 Manipulation of TPN caloric substrate and fatty infiltration of liver. J Surg Res 31: 46–54
Vileisis RA, Inwood RJ, Hunt CE 1980 Prospective controlled study of parenteral nutrition associated cholestatic jaundice: effect of protein intake. J Pediatr 96: 893–897
Stein TP, Buzby G, Hargrove WC, Leskiw MJ, Mullen JL 1980 Essential fatty acid deficiency in patients receiving simultaneous parenteral and oral nutrition. JPEN 4: 343–345
Freund HR, Rimon B 1990 Sepsis during total parenteral nutrition. JPEN 14: 39–41
Wolf A, Pohlandt F 1989 Bacterial infection: the main cause of acute cholestasis in newborn infants receiving short-term parenteral nutrition. J Pediatr Gastroenterol Nutr 8: 297–303
Shu Z, Li J, Zhou Z, Shi Q, Zhang T 1991 Histopathologic study of cholestasis induced by total parenteral nutrition or intraperitoneal sepsis in rats. JPEN 15: 630–636
Bhatia J, Rivera A, Moslen MT, Rassin DK, Gourley WK 1992 Hepatic function during short-term total parenteral nutrition: effect of exposure of parenteral nutrients to light. Res Commun Chem Pathol Pharmacol 78: 321–340
Bhatia J, Stegink LD, Ziegler EE 1983 Riboflavin enhances photo-oxidation of amino acids under simulated clinical conditions. JPEN 7: 277–279
Bhatia J, Treinen M, Haque AK, McCleery R, Rassin DK 1993 Total parenteral nutrition-associated alterations in hepatobiliary function and histology in rats: is light exposure a clue. Pediatr Res 33: 487–492
Ke J, Tam YK, Koo WWK, Gray MR, Coutts RT 1990 Effects of parenteral nutrition on hepatic elimination of lidocaine: a study using the isolated perfused rat liver. J Pharmacol Exp Ther 255: 351–356
Tam YK, Yau R, Bergins R 1987 Mechanisms of lidocaine kinetics in the isolated perfused rat liver 1. Effects of continuous infusion. Drug Metab Dispos 15: 12–16
Miller LL 1973 Technique of isolated rat liver perfusion. In: Bartosek I, Guaitani A, Miller LL (eds) Isolated Liver Perfusion and Its Applications. Raven Press, New York, pp 11–52
Hussain MD, Tam YK, Gray MR, Coutts RT 1994 Mechanisms of time-dependent kinetics of diltiazem in the isolated perfused rat liver. Drug Metab Dispos 22: 36–42
Bloxam DL 1973 Condition and performance of the rat liver perfused with Kreb's ringer solution, with particular reference to amino acid metabolism. In: Bartosek I, Guaitani LL, Miller LL (eds) Isolated Liver Perfusion and Its Applications. Raven Press, New York, pp 147–157
Gores GJ, Kost LJ, LaRusso NF 1986 The isolated perfused rat liver: Conceptual and practical considerations. Hepatology 6: 511–517
Sedgwick GW, Fenton TW, Thopmson JR 1991 Effect of protein precipitating agents on the recovery of plasma free amino acids. Can J Anim Sci 71: 953–957
Kleinman LM, Tangrea JA, Gallelli JF 1973 Stability of solutions of essential amino acids. Am J Hosp Pharm 30: 1054–1057
Kanner JD, Fennema OJ 1987 Photooxidation of tryptophan in the presence of riboflavin. Agric Food Chem 35: 71–76
Gurnani S, Arifuddin M, Augusti KT 1966 Effect of visible light on amino acids. 1. Tryptophan, Photochem Photobiol 5: 495–505
Asquith RS, Rivett DE 1971 Studies on the photooxidation of tryptophan. Biochim Biophys Acta 252: 111–116
Gurnani S, Arifuddin M 1966 Effect of visible light on amino acids-11. Histidine. Photochem Photobiol 5: 341–345
Nelson SD, Garland WA, Breck GD, Trager WF 1977 Quantification of lidocaine and several metabolites utilizing chemical-ionization mass spectrometry and stable isotope labeling. J Pharm Sci 66: 1180–1190
Ngo LY, Tam YK, Coutts RT 1995 Lack of residual effects diethyl ether, methoxyflurane and sodium pentobarbital on lidocaine metabolism in a single-pass isolated rat liver perfusion system. Drug Metab Dispos 23: 525–528
Saville BA, Gray MR, Tam YK 1989 Evidence for lidocaine-induced inactivation. J Pharm Sci 78: 1003–1008
Norusis MJ 1992 SPSS for windows base system user's guide [computer program]. SPSS Inc., Chicago
Montgomery DC 1991 Nonparametric methods in the analysis of variance. In: Montgomery DC (ed) Design and Analysis of Experiments. John Wiley & Sons, New York, pp 126–133
1984 Guide to the care and use of experimental animals In: Canadian Council on Animal Care. Ottawa, 84 pp
Donoso MLN, Valenzuela A, Silva E 1988 Tryptophan-riboflavin photo-induced adduct and hepatic dysfunction in rats. Nutr Rep Int 37: 599–606
Bhatia J, Rassin DK, McAdoo DJ 1991 Photosensitized oxidation of tryptophan: Effect on liver and brain tryptophan. JPEN 15: 637–641
Oda Y, Imaoka S, Nakahira Y, Asada A, Fujimori M, Fujita S, Funae Y 1989 Metabolism of lidocaine by purified rat liver microsomal cytochrome P-450 isozymes. Biochem Pharmacol 38: 4439–4444
Imaoka S, Enomoto K, Oda Y, Asada A, Fujimori M, Shimada T, Fujita S, Guengerich FP, Funae Y 1990 Lidocaine metabolism by human cytochrome P-450s purified from hepatic microsomes: comparison of those with rat hepatic cytochrome P-450s. J Pharmacol Exp Ther 255: 1385–1391
Bargetzi MJ, Aoyama T, Gonzalez FJ, Meyer UA 1989 Lidocaine metabolism in human liver microsomes by cytochrome P450IIIA4. Clin Pharmacol Ther 46: 521–527
Masubuchi Y, Araki J, Narimatsu S, Suzuki T 1992 Metabolic activation of lidocaine and covalent binding to rat liver microsomal protein. Biochem Pharmacol 43: 2551–2557
Coutts RT, Su P, Baker GB 1994 Involvement of CYP2D6, CYP3A4, and other cytochrome P-450 isozymes in N-dealkylation reactions. J Pharmacol Toxicol Methods 31: 177–186
Smith DA 1991 Species differences in metabolism and pharmacokinetics: are we close to an understanding. Drug Metab Rev 23: 355–373
Cholerton S, Daly AK, Idle JR 1992 The role of individual human cytochrome P-450 in drug metabolism and clinical response. Trends Pharmacol Sci 13: 434–439
Zaman N, Tam YK 1994 Effects of intralipid as a source of energy in parenteral nutrition on lidocaine elimination: a study using isolated rat liver perfusion. Pharm Res 11:S407.
Zaman N, Tam YK 1993 Effects of cholestyramine and total parenteral nutrition on hepatic elimination of lidocaine: a study using isolated rat liver perfusion. Pharm Res 10:S361.
Knodell RG, Steele NM, Cerra FB 1984 Effects of parenteral and enteral hyperalimentation on hepatic drug metabolism in the rat. J Pharmacol Exp Ther 229: 589–597
Ross LH, Griffeth L, Hall RI, Bozovic MG, Rauckman E, Grant JP 1983 Hepatotoxic effects of parenteral nutrition upon in vivo pharmacokinetics of antipyrine. Surg Forum 34: 34–36
Rivera A, Bhatia J, Rassin KR, Gourley WK, Catarau E 1989 In vivo biliary function in the adult rat: the effect of parenteral glucose and amino acids. JPEN 13: 240–245
Hartshorn RD, Demers LM, Sultatos LG 1979 Effect of chronic parenteral carbohydrate administration on hepatic drug metabolism in the rat. Pharmacology 18: 103–111
Pantuck EJ, Pantuck CB, Weissman C 1995 Effects of parenteral nutrition regimens on oxidative drug metabolism. Anaesthesiology 60: 534–536
Gimmon Z, Nachbauer C, Fischer JE 1981 Comparative efficacy of parenteral vs enteral nutrition in the post-traumatic rat. Gastroenterology 80: 1156
Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, Soeters PB 1976 The effect of normalization of plasma amino acids on hepatic encephalopathy. Surgery 80: 77–91
Fischer JE, Funovics JM, Aguirre A, James JH, Keane JM, Wesdorp RI, Yoshimura N, Westman T 1975 The role of plasma amino acids in hepatic encephalopathy. Surgery 78: 276–290
Freund H, Atamian S, Holroyde J, Fischer JE 1979 Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg 190: 571–576
Burgess P, Hall RI, Bateman MD 1987 The effect of total parenteral on hepatic drug oxidation. JPEN 11: 540–543
Bhatia J, Moslen MT, Kaphalia L, Rassin DK 1992 Glutathione and tissue amino acid responses to light-exposed parenteral nutrients. Toxicol Lett 63: 79–89
Bhatia J, Mims LC, Roesel RA 1980 The effect of phototherapy on amino acid solutions containing multivitamins. J Pediatr 96: 284–286
Weil L, Gordon WG, Buchart AR 1951 Photooxidation of amino acids in the presence of methylene blue. Arch Biochem Biophys 33: 90–109
Litwin J 1972 The effect of light on the aging of human diploid fibroblasts. Exp Gerontol 7: 381–386
Nixon TB, Wang RJ 1977 Formation of photoproducts lethal for human cell in culture in daylight fluorescent light and bilirubin light. Photochem Photobiol 26: 589–591
Hirata Y, Kawachi T, Sugimura T 1967 Fatty liver induced by injection of tryptophan. Biochim Biophys Acta 144: 233–241
Heger J, Frydrych Z 1989 Efficiency of utilization of amino acids In: Friedman M (ed) Absorption and Utilization of Amino Acids. CRC Press, Boca Raton, FL, pp 31–56
Author information
Authors and Affiliations
Additional information
Supported by Medical Research Council of Canada.
Rights and permissions
About this article
Cite this article
Zaman, N., Tam, Y., Jewell, L. et al. Effects of Light-Exposed Parenteral Nutrition on Hepatic Function and Lidocaine Metabolism: A Study Using Isolated Rat Liver Perfusion. Pediatr Res 40, 280–287 (1996). https://doi.org/10.1203/00006450-199608000-00015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199608000-00015