Abstract
Bilirubin encephalopathy results from the entry of bilirubin into the brain and is expressed by motor, sensory, and/or behavioral impairment. The jaundiced (jj) Gunn rat is a valuable animal model for studying the kinetics of bilirubin-induced neurotoxicity. This is often done by recording evoked potentials, which are also used as indices of brain damage in infants who develop neonatal jaundice, as is the case with the auditory nerve and brainstem evoked response (ABR). The present study describes the postnatal development of the somatosensory evoked potential (SEP) in Gunn rats. No effects of jaundice on the SEP were found in young jj rats (16-28 d). However, adult (3-4 mo) jj rats had prolonged latencies and decreased amplitudes of the P2 component of the SEP compared with adult nonjaundiced (Jj) rats. These changes in the SEP of jaundiced rats may reflect a synaptic lesion in these animals, possibly due to cumulative and/or progressive damage induced by bilirubin during the first 3 mo of life. After sulfadimethoxine administration, marked latency prolongations (2-6%) were observed in the early components of SEP in young (3-wk-old) jj (but not Jj) rats, as early as 2 h after injection. These changes, which became more severe (4-10%) with time, seem to be mostly peripheral. The present results suggest that the SEP may be a sensitive marker for the massive entry of bilirubin into the nervous system, and could serve as part of an evoked potential battery (in addition to visual evoked potential and ABR) in assessing bilirubin-induced neurotoxicity in jaundiced newborns and infants.
Similar content being viewed by others
Main
Bilirubin encephalopathy, which results from the entry of bilirubin into the brain, may lead to impairment of motor, sensory (e.g. hearing loss) and behavioral functions. Research in this field has greatly benefited from the availability of a suitable experimental animal model-the homozygous recessive, jj Gunn rat. These rats develop unconjugated hyperbilirubinemia from birth, with plasma bilirubin levels reaching a peak (≈205 μmol/L) at about 2 wk and then slowly declining. The average plasma bilirubin level in the adult jj rats is ≈171 μmol/L(1). About 20-30% of the jj rats in our colony do not survive beyond the first month of life.
Evoked potentials have been recorded in Gunn rats to follow the functional manifestations of bilirubin encephalopathy and investigate its pathogenesis. ABR abnormalities were reported to be minimal in developing jj rats(2). Moreover, ABR was found to be normal in adult jj rats, as were the ABR thresholds(2, 3). The VEP has also been studied, with the hope that it would serve as a more sensitive marker for bilirubin-induced neurotoxicity in the cortex. Marked VEP abnormalities were demonstrated in 3-wk-old jj rats compared with heterozygous, Jj rats(4). On the other hand, the VEP was found to be normal in adult jj rats, as was the case with the ABR. The VEP abnormalities in young animals were not correlated with plasma bilirubin levels or body weight. The 21-d-old jj rats had significantly lower body weights (27.9 ± 3.1 g) than the Jj rats (31.8 ± 1.6 g).
The SEP is an additional evoked response modality which reflects both peripheral and central activity of the somatosensory pathway(5–7). The SEP has been shown to be a sensitive marker of motor impairment(8, 9). Such damage is also characteristic of bilirubin encephalopathy in jaundiced neonates, in whom it is manifested by extrapyramidal signs (e.g. cerebral palsy, ataxia, and tremor)(10, 11). The SEP has been therefore recorded in infants during and after hyperbilirubinemia, and abnormalities were demonstrated in this evoked response(12).
The present study was designed to determine whether the SEP would be affected by hyperbilirubinemia during development of jj rats, as well as by acute shifting of bilirubin from the vascular compartment into the nervous system after administration of SDM(13–15). SDM produced marked changes in VEP(16) and ABR(17) of young jj rats, as early as 2 h after injection of the drug.
METHODS
Animals. Female Jj rats from our colony at the Hebrew University-Hadassah Medical School were mated with jj males. The offspring were kept with the mother for 22 d, at a constant room temperature(23-25°C) and a 12-h light-12-h dark cycle. The animals were fed rat chow containing 11% fat (Weizmann Institute, Rehovot, Israel) and water ad libitum.
SEP recordings were obtained from the survivors of an original group of 17 jj pups (originating from four litters) at the ages of 16, 18, 21, and 28 d, and compared with those obtained from 14 Jj littermates. Five out of the 17 jj rats died during the 1st mo of life (four out of these five animals died during the 4th wk of life), and three did not survive into adulthood. SEP was also recorded in 23 adult (3-4 mo-old) jj rats (nine survivors of the original group tested during the 1st mo of life and 14 additional rats) and in 19 adult Jj rats (13 from the original group, and six additional rats). Because there were no statistical differences in the average values of latency and amplitude between the two subgroups (original and additional rats) of jj animals, these subgroups were combined for presentation of results, and thus sample size was increased. For similar reasons, the two subgroups of Jj rats were also combined.
SDM administration. SDM sodium salt (Sigma Chemical Co., St. Louis, MO) was dissolved in saline (20 mg/mL) and injected intraperitoneally(100 mg/kg) into 3-wk-old jj (n = 22) and Jj (n = 20) rats, immediately after obtaining initial baseline SEP recordings. SEP was again recorded 2 and 6 h after injection.
Blood samples were taken from the tail vein immediately after baseline recordings (before injection) and then again after the final recording (6 h). Plasma bilirubin levels were determined by the method of Michaelsson(18).
SEP recordings. Rats were anesthetized by ether inhalation. Body temperature was monitored with a tele-thermometer (Yellow Springs Instrument Co., Yellow Springs, OH) and maintained at 36-37°C with a heating pad. The SEP was elicited by 0.05-ms current pulses delivered by needle electrodes (Grass Instruments, Quincy, MA) to the forepaw, with an intensity sufficient to produce a just detectable twitch. The early components(compound action potentials) of the SEP were recorded by placing needle electrodes in the skin at the contralateral (to stimulated forepaw) scalp parietal region and at the upper part of the ipsilateral forelimb, using a stimulus rate of 4/s and a recording filter bandpass optimal for recording compound action potentials (200-2000 Hz; n = 256). The later, slower, cortical components of the SEP were recorded by placing electrodes at the contralateral parietal region and chin (stimulus rate 2/s; filter bandpass 30-3000 Hz; n = 512). Based on earlier experimentation, these stimulus rates, number of stimuli, and recording filter bandpasses were found to be optimal for recording these components both in neonatal and adult rats. A ground electrode was placed at a hind limb, SEP was recorded with a Micro-Shev C-ERA 100 evoked response system (Micro-Shev, Efrat, Israel). At least two traces were recorded for each condition. Latencies and peak to peak amplitudes were presented as mean ± SD, and significance was evaluated using repeated measures analysis of variance.
RESULTS
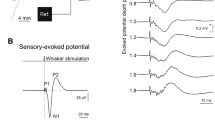
Early components of SEP. The first of the early SEP components was a scalp positive P1 wave, followed by waves N1, P2, N2(Fig. 1A). Early components of SEP could be recorded only from d 16 in all the rats, except one Jj rat in which they appeared at a later age.
The latencies of all four SEP components became shorter between the ages of 16 d and 3 mo (Fig. 2). No significant differences in SEP wave latencies were found between young (16-28 d old) jj and Jj rats. The four jj rats who died during the 4th wk of life did not differ significantly from their surviving jj littermates with respect to wave latencies, neither on d 18 nor on d 21. However, the adult jj rats exhibited a prolonged wave P2 latency(2.53 ± 0.16 ms) compared with the adult Jj rats (2.33 ± 0.17 ms) (p < 0.0005) (Fig. 2).
The amplitudes of waves N1 and P2 tended to increase slightly between 16 and 28 d of age in both jj and Jj rats (Fig. 3), whereas changes in wave P1 amplitude were inconsistent. Between the age of 28 d and adulthood, there were marked increases in the amplitudes of all three waves(P1, N1, and P2) in both jj and Jj rats. Adult jj rats exhibited a significantly (p < 0.05) lower wave P2 amplitude (2.32 ± 0.81 μV) compared with the adult Jj animals (3.35 ± 1.88 μV)(Fig. 3), whereas no differences were found in wave amplitudes between the young jj and Jj animals.
Later (cortical) components of SEP. The later SEP component was a P-N wave (Fig. 1B), which was not apparent in most of the animals until the 4th wk of life. At this age, the P-N wave was present in only a few animals and was very broad, making latency and amplitude determinations difficult. When comparing cortical SEP in adult jj and Jj rats, no differences were found either in peak latencies (8.51 ± 0.47 and 8.32 ± 0.49 ms, respectively) or in amplitudes (7.31 ± 5.09 and 7.77 ± 3.26 μV, respectively). Interestingly, no cortical components could be detected in one adult jj rat.
Effects of SDM. SEP was recorded in a group of 22 jj and 20 Jj rats, 19-23 d old, originating from eight litters. All of the rats had, before injection, early components of SEP typical for this age (Fig. 4). Plasma bilirubin levels in jj rats before SDM administration were 239± 41 μmol/L. Six h after injection, plasma bilirubin levels markedly decreased to 51 ± 19 μmol/L (p < 0.0001). Two hours after SDM injection, marked latency prolongations of waves P1 and N2 were already observed in jj rats, compared with their latencies before injection. Six hours after injection, jj rats exhibited marked latency prolongations for all four early components of SEP (Figs. 4 and5). Mean changes in SEP latencies after SDM administration are summarized inTable 1. Note that the increments in latency were similar for all four waves-P1, N1, P2, and N2. However, in one of the jj rats only wave P1 could be detected 2 h after injection, whereas 6 h after injection there were no clear SEP waves (Fig. 4B). In Jj rats, there were no significant changes in wave latencies 2 h after injection, whereas 6 h after injection a tendency for shortening of the latencies of waves N1, P2, and N2 was noted (Fig. 5). Only the amplitude of wave N1 was significantly decreased (p < 0.02) in jj rats, 2 h after injection of SDM. In Jj rats, no significant changes in SEP wave amplitudes could be demonstrated after SDM administration.
Changes in SEP in two jj rats after SDM administration. In rat A, marked prolongations of wave latencies were apparent as early as 2 h after injection. In rat B, only wave P1 could be detected 2 h after SDM injection, whereas no clear SEP waves could be detected 6 h after this injection. S indicates the time of stimulation. Positivity at the scalp electrode is displayed upward.
DISCUSSION
Because the jaundiced Gunn rat is ataxic and SEP changes have been found to accompany motor impairment (e.g. ataxia) in humans(8, 9), SEP was recorded in these rats to determine the possible effects of neonatal hyperbilirubinemia on the postnatal development of this evoked potential. However, no clear SEP changes could be demonstrated in young jj rats even though they already showed signs of ataxia. On the other hand, unlike the VEP(4) or the ABR(2, 3), there were clear SEP changes in the adult jaundiced rats. This conclusion was based on the significantly prolonged latency and decreased amplitude of wave P2 observed in adult jj as compared with adult Jj rats. This component of the SEP is probably generated in the medulla, after the first synapse in the somatosensory pathway (dorsal column nuclei)(5–7). A synaptic lesion due to bilirubin toxicity has been previously demonstrated in different parts of the central nervous system, such as the cochlear nuclei(19) and the hippocampus(20). On the other hand, there is some evidence that the P2 component of the SEP may be generated in the upper part of the spinal cord, before the first synapse of this sensory pathway(21). It is therefore not clear whether these changes in P2 reflect a synaptic or axonal lesion at this level. In addition, it should be mentioned that a spinal cord lesion was found in autopsies of children with a history of hyperbilirubinemia and kernicterus(22). It is unlikely that this abnormality of P2 in 3-mo-old jj rats is due to malnutrition (jj weigh less than Jj rats), because experimental undernutrition in neonatal rats induced only transient ABR changes in young rats. These changes were no longer present in 33-d-old rats(23).
The clear SEP changes observed in adult Gunn rats, compared with the lack of subcortical SEP changes in developing rats, may result from cumulative, progressive damage after a massive early entry or a slow and continuous penetration of bilirubin into the nervous system. In this respect, the present results differ from those obtained for the cortical VEP in young jj rats during development(4). This may reflect a different susceptibility of cortical and subcortical regions of the nervous system to bilirubin toxicity. However, the ABR is also generated in subcortical areas of the auditory pathway but, unlike the early components of SEP, some ABR abnormalities were demonstrated in developing jj rats(2). Furthermore, SEP changes could be demonstrated in the jaundiced animals only as adults, whereas VEP and ABR were usually normal in these animals.
It is possible that SEP changes would be observed in young jj rats, if they were exposed to higher plasma bilirubin levels for longer periods of time. Such conditions could have prevailed in hyperbilirubinemic newborns in whom SEP abnormalities were reported. Some of these abnormalities were progressive, whereas others occurred later in time and not during, or immediately after, the hyperbilirubinemic period(12). Thus, even though no SEP changes were seen in developing jj rats, we have observed that SDM, which shifts bilirubin from plasma into neural and other extravascular compartments(13–15), led to severe abnormalities in the early components of SEP in 3-wk-old jj rats. These abnormalities were apparent as early as 2 h after injection of SDM and became more severe with time. Moreover, the full extent of this damage became apparent immediately, in contrast to the damage seen during development in these rats, in which it became apparent only at the age of 3-4 mo.
The marked changes in wave P1 latency in the SDM-treated jj rats probably reflect a peripheral lesion in the somatosensory pathway, because this component of the SEP is generated in the peripheral nerve, before the first synapse(5–7). In addition, the effects that were demonstrated in the SDM-treated jj rats were seen for all the early components of the SEP, similar to those reported for hyperbilirubinemic newborns(12). However, the damage seems to be peripheral, because there was no evidence for further change in later waves, greater than that seen for wave P1 (Table 1). In contrast, in the adult jj rats included in the developmental study, the only effect observed was on wave P2, which is probably a postsynaptic component(5–7). It is possible that a different type of lesion results from the short-term, rapid, and massive increase in brain bilirubin content (as occurs in the SDM experiment), compared with the lesion due to the long-term, “chronic,” exposure to bilirubin (as is the case during development). Nevertheless, differences in vulnerability of the somatosensory pathway in young rats, compared with adult rats, cannot be excluded. We cannot explain at this moment the tendency toward shorter latencies of waves N1, P2, and N2 after SDM injection in Jj rats, a tendency that is similar to that observed in VEP recordings in Jj rats after SDM administration(16).
A clear cortical SEP was not detected in the developing Gunn rats. This was not due to the choice of stimulus rate and recording filter bandpass, because these were suitable for recording the cortical SEP in adult rats, and attempts to use other repetition rates and bandpasses in young rats were not successful. It was also probably not due to the ether anesthesia, because the VEP (also a cortical response) in young etheranesthetized Gunn rats(4) was similar to that recorded in awake, unsedated rats(24). In adult Gunn rats, a cortical SEP response was detected, but there were no differences between jj and Jj rats, even though the P2 early wave was delayed in latency and reduced in amplitude in the adult jj animals. However, a 0.2-ms prolongation in P2 latency would probably not be apparent in the late (cortical) recording considering the time resolution used. Cortical SEP has been recorded in human newborns(8) and may be contributory in cases of neonatal jaundice, because motor impairment, one of the sequelae of bilirubin encephalopathy(10, 11), was found to be highly correlated with cortical SEP abnormalities(8).
In conclusion, it appears that it may be advisable to complement the evoked potentials test battery with the SEP (in addition to the ABR and VEP) for the evaluation of infants with hyperbilirubinemia. Although the ABR and VEP can serve as diagnostic tools during the first weeks of life, the SEP may add more information about late and/or progressive changes and may therefore have an important prognostic value in jaundiced infants. Furthermore, this evoked potentials test battery may further contribute to the analysis of the mechanism of bilirubin-induced encephalopathy.
Abbreviations
- SEP:
-
somatosensory evoked potential
- VEP:
-
visual evoked potential
- ABR:
-
auditory nerve and brainstem evoked response
- jj:
-
jaundiced Gunn rat
- Jj:
-
nonjaundiced Gunn rat
- SDM:
-
sulfadimethoxine
REFERENCES
Kapitulnik J, Gonzalez FJ 1993 Marked endogenous activation of the CYP1A1 and CYP1A2 genes in the congenitally jaundiced Gunn rat. Mol Pharmacol 43: 722–725
Shapiro SM, Hecox KE 1988 Development of brainstem auditory evoked potentials in heterozygous and homozygous jaundiced Gunn rats. Dev Brain Res 41: 147–157
Lenhardt ML, Clarke AM, Harkins SW 1986 High frequency hearing in jaundiced rats. J Aud Res 26: 19–25
Silver S, Kapitulnik J, Sohmer H 1991 Postnatal development of flash visual evoked potentials in the jaundiced Gunn rat. Pediatr Res 30: 469–472
Allison T, Hume AL 1981 A comparative analysis of short-latency somatosensory evoked potentials in man, monkey, cat and rat. Exp Neurol 72: 592–611
Hashimoto I 1984 Somatosensory evoked potentials from the human brain-stem: origins of short latency potentials. Electroencephalogr Clin Neurophysiol 57: 221–227
Nakanishi T, Tamaki M, Arasaki K, Kudo N 1982 Origins of the scalp recorded somatosensory far field potentials in man and cat. Electroencephalogr Clin Neurophysiol 36 ( suppl): 336–338
Taylor MJ, Murphy WJ, Whyte HE 1992 Prognostic reliability of somatosensory and visual evoked potentials of asphyxiated term infants. Dev Med Child Neurol 34: 507–515
Nonsiainen U, Partanen J, Laulumaa V, Paakkonen A 1987 Involvement of somatosensory and visual pathways in late onset ataxia. Electroencephalogr Clin Neurophysiol 67: 514–520
Hyman CB, Keaster J, Hansen V, Harris I, Sedgwick R, Wursten H, Wright AR 1969 CNS abnormalities after neonatal hemolytic disease or hyperbilirubinemia. Am J Dis Child 117: 395–405
Oski FA 1991 Kernicterus. In: Taeusch HW, Ballard RA, Avery ME (eds) Schaffer and Avery's Diseases of the Newborn. WB Saunders, Philadelphia, pp 760–762
Bongers-Schokking CJ, Colon EJ, Hoogland RA, Van Den Brande JLV, De Groot CJ 1990 Somatosensory evoked potentials in neonatal jaundice. Acta Paediatr Scand 79: 148–155
Oie S, Levy G 1979 Effect of sulfisoxazole on pharmacokinetics of free and plasma protein-bound bilirubin in experimental unconjugated hyperbilirubinemia. J Pharmacol Sci 68: 6–9
Bratlid D, Cashore WJ, Brubakk AM, Oh W 1984 Bilirubin displacement by sultisoxazole: entry of unbound bilirubin into the brain. Pediatr Res 18( suppl): 150A.
Robertson A, Karp W, Brodersen R 1991 Bilirubin displacing effect of drugs used in neonatology. Acta Paediatr Scand 80: 1119–1127
Silver S, Sohmer H, Kapitulnik J 1995 Visual evoked potential abnormalities in jaundiced Gunn rats treated with sulfadimethoxine. Pediatr Res 38: 258–261
Shapiro SM 1988 Acute brainstem auditory evoked potential abnormalities in jaundiced Gunn rats given sulfonamides. Pediatr Res 23: 306–310
Michaelsson M 1961 Bilirubin determination in serum and urine: studies on diazomethods and a new copper-azopigment method. Scand J Clin Lab Invest I ( suppl): 46–47
Zhang S, Wickesberg RE, Oertel D 1989 Jaundiced Gunn rats have increased synaptic delays in the ventral cochlear nucleus. Brain Res 501: 194–197
Hansen TWR, Paulsen O, Gjerstad L, Bratlid D 1988 Short-term exposure to bilirubin reduces synaptic activation in rat transverse hippocampal slices. Pediatr Res 23: 453–456
Moller AR, Jannetta PJ, Burgess JE 1986 Neural generators of the somatosensory evoked potentials: recordings from the cuneate nucleus in man and monkeys. Electroencephalogr Clin Neurophysiol 65: 241–248
Zimmerman HM, Yannet H 1935 Cerebral sequelae of icterus gravis neonatorum and their relation to kernicterus. Am J Dis Child 49: 418–430
Kawai S, Nakamura H, Matsuo T 1989 Effects of early postnatal undernutrition on brainstem auditory evoked potentials in weanling rats. Biol Neonate 55: 268–274
Rigdon GC, Dyer RS 1987 Ontogeny of flash-evoked potentials in unanesthetized rats. Int J Dev Neurosci 5: 447–454
Author information
Authors and Affiliations
Additional information
Part of a Ph.D. thesis submitted by S.S. to the Senate of the Hebrew University in Jerusalem.
Rights and permissions
About this article
Cite this article
Silver, S., Sohmer, H. & Kapitulnik, J. Postnatal Development of Somatosensory Evoked Potential in Jaundiced Gunn Rats and Effects of Sulfadimethoxine Administration. Pediatr Res 40, 209–214 (1996). https://doi.org/10.1203/00006450-199608000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199608000-00005