Abstract
The effect of hyperglycemia on ischemic brain damage was investigated in a newborn dog model of hypothermic circulatory arrest. Newborn dogs were anesthetized with halothane, paralyzed, and artificially ventilated to maintain normoxia and acidbase balance. Animals were surface-cooled to 20°C, after which cardiac arrest was effected with i.v. KCl. Before surface cooling, one-half of the dogs (n = 12) received a bolus injection of 50% glucose to increase plasma glucose concentrations to approximately 33 mmol/L (600 mg/dL); control littermates (n = 12) received an equivalent volume of 1 N saline. The dogs remained asystolic for 1.75 h, after which cardiopulmonary resuscitation was accomplished. All animals survived, were allowed to recover from anesthesia at 37°C, and were maintained for 8 h of recovery, at which interval they underwent perfusion-fixation of their brains for pathologic analysis. Histologic grading of brain damage showed no statistically significant difference in the severity of neuronal necrosis within the cerebral cortex or caudate nucleus between hyperglycemic and normoglycemic littermates, with greater brain damage apparent in the amygdaloid nucleus of the hyperglycemic dogs (p < 0.02). Brainstem injury occurred more frequently in the hyperglycemic animals(p < 0.05). Correlation of coefficients analyses revealed a positive correlation between the severity of brain damage and plasma glucose concentration for both the caudate nucleus and amygdaloid nucleus but not for the cerebral cortex. The findings suggest that hyperglycemia superimposed upon hypothermic circulatory arrest in the newborn dog accentuates brain damage only in selected regions of the brain, especially the caudate and amygdaloid nuclei and brainstem, excluding the cerebral cortex.
Similar content being viewed by others
Main
Hypothermic circulatory arrest has become an established procedure for the surgical correction of congenital heart anomalies in human infants(1–3). Infants usually can tolerate up to 70 min of total circulatory arrest at core temperatures ranging from 16 to 24°C without sustaining ischemic brain damage(4–6). Unfortunately, infants occasionally sustain brain damage even when the duration of cardiac arrest is well within the “therapeutic window”(1, 7). It has not been established why such infants are more susceptible than other similarly treated infants to ischemia-induced neuronal injury. Many biologic variables might be critical for brain damage to occur, one of which would be the level of circulating glucose before or after the ischemic phase. In this regard, investigations in adult animals and humans indicate that hyperglycemia accentuates ischemic brain damage(8–11). Because hyperglycemia might contribute to the occurrence of brain damage arising from hypothermic circulatory arrest in human infants, we designed an experiment in a newborn animal model to address this issue.
METHODS
Animal preparation. Pregnant mongrel dogs were purchased from a local breeder and housed in individual kennels. After spontaneous vaginal delivery, the newborn puppies were kept with their bitches until the time of experimental manipulation at 3-5 d of postnatal age. The newborn dogs were anesthetized with halothane (4% induction; 1.0-1.5% maintenance), after which they underwent endotracheal intubation and muscular paralysis with succinylcholine (15 mg/kg of body weight). Thereafter, the animals were artificially ventilated with a gas mixture of 0.5% halothane-70% nitrous oxide-29.5% oxygen. Under local anesthesia (1% procaine HCl), a femoral artery was cannulated with polyethylene tubing (PE-50), which was connected via a Statham transducer (Gould, Oxnard, CA) to a dynographic recorder (model R711; Beckman Instruments, Fullerton, CA) to monitor systemic heart rate and blood pressure. A side arm of the catheter allowed for intermittent collection (0.2 mL) of arterial blood for analysis of oxygen and acid-base status on a micro-blood gas analyzer (model ABL 30; Radiometer America, Westlake, OH). Oyxgen and acid-base balance was maintained within a narrow range[Paco2, 4.4-5.6 kPa (35-42 mm Hg); pHa, 7.35-7.42; Pao2,>8.0 kPa (60 mm Hg)] by small adjustments of tidal volume and ventilatory rate. Paco2, Pao2, and pHa were measured at 37.0°C during both normothermia and hypothermia. Plasma glucose was measured intermittently on a micro-glucose analyzer (Glucostat; Beckman Instruments). A femoral vein was also cannulated as a route for injections of drugs and glucose. Body temperature was monitored by means of a rectal probe attached to a servo-controlled heating lamp and was initially maintained at 37 ± 0.2°C.
Induction of hypothermia. Once steady state arterial normoxia and acid-base balance were achieved, one newborn dog of a pair received a bolus i.v. injection over 5 min of 50% glucose (5.0 mL/kg); the littermate control received an equivalent volume of 1 N saline. No change in blood pressure or heart rate occurred after the glucose injection, which suggests the absence of any osmolar shifts during or after the glucose administration. Immediately thereafter, each puppy was gently positioned prone on a plastic bag containing crushed ice, which extended around the sides of the animal. Ice was not applied to the midline portion of the back or to the head. Rectal temperature was continuously monitored during the cooling period, and the ice packs were removed when the temperature reached 20°C. Thereafter, the temperature was maintained within a narrow range (±0.5°) by either reapplying the ice packs near to the side of the animal or by automatic activation of the heating lamp. No adjustments in tidal volume or ventilatory rate were made during or after the cooling process. The interval of cooling necessary to lower body temperature from 37 to 20°C ranged from 60 to 90 min. A previous investigation has demonstrated that, once a stable level of systemic hypothermia is achieved for 30 min, brain temperature remains 1-2°C above rectal temperature(12).
Circulatory arrest and resuscitation. Once hypothermia to 20°C was achieved for 30 min, the heart of each newborn dog was arrested with the i.v. injection of KCl (25 mmol/L) in a small volume (2-3 mL). Artificial ventilation was discontinued simultaneously with the cardiac arrest. Complete circulatory arrest was verified by the absence of spontaneous heart rate and systemic blood pressure monitored on the dynograph. All animals remained asystolic for 1.75 h, an interval adequate to produce brain damage in all animals(13–15). Thereafter, the animals were resuscitated by: 1) resumption of artificial ventilation with 70% nitrous oxide-30% oxygen at the prearrest rate without change in tidal volume; 2) body warming with the heating lamp; 3) i.v. injection of NaHCO3 (2.0 mmol/kg); 4) i.v. injection of 1/10,000 epinephrine (0.02 mg/kg); and 5) closed chest cardiac massage at a rate of 60 compression/min. These maneuvers resulted in spontaneous heart action in all animals, with progressively increasing heart rate and systemic blood pressure thereafter. Plasma glucose, hematocrit, arterial oxygen, and acid-base status were determined at 15 min; 1, 2, 4, and 8 h of recovery. Once the plasma glucose returned to the normoglycemic range(5-8 mmol/L), the animals received a variable infusion of 10% glucose in water to maintain normoglycemia. Metabolic acidemia (pHa < 7.25) was treated with intermittent bolus injections of NaHCO3 (1.0 mmol/kg). Hypocapnia (Paco2 < 4.0 kPa) was corrected by adjustments in ventilatory rate or tidal volume. Once rewarming to 37°C was complete, the animals were weaned from the ventilator as the effect of the succinylcholine subsided. Once a stable, spontaneous respiratory pattern was achieved, the endotracheal tube was removed. Thereafter, the puppies were placed in an infant warmer with an environmental temperature of 34.0°C and continued to receive an i.v. infusion of 10% glucose in water to maintain optimal glucose and fluid homeostasis. The animals were maintained for 8 h, at which time they underwent perfusion-fixation of their brains. It has been demonstrated previously that, by 8 h of recovery, brain damage in this model is well advanced and that delayed neuronal necrosis does not occur(13–15).
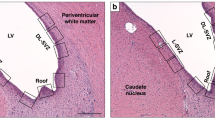
Neuropathologic methods. At 8 h of recovery from hypothermic circulatory arrest, each newborn dog was deeply anesthetized with pentobarbital (50 mg/kg i.v.), at which time they underwent perfusion-fixation of their brains with formaldehyde-glacial acetic acid-absolute methanol(1:1:8) for 30 min, as previously described(13–15). At the completion of the perfusion-fixation, the scalp was removed and the brain together with the entire head was fixed an additional 24 h in formaldehyde-glacial acetic acid-absolute methanol, after which it was removed entirely from the skull and fixed in FAM for 1 wk. Each formaldehyde-glacial acetic acid-absolute methanol-fixed brain was cut coronally in 2-3-mm thick slices, which were individually embedded in paraffin. Sections (6 μm thick) were stained with hematoxylin and eosin and examined blindly (J.T.) at the light microscopic level.
To ascertain quantitatively the extent of neuronal necrosis, the populations of damaged neurons/1 mm2 area of cerebral cortex, caudate nucleus, and amygdaloid nucleus were determined. These regions of brain were chosen because of their known vulnerability to ischemic damage in the newborn dog(13–15). The method of damaged neuronal counting previously has been described in detail(14).
Statistical analyses. All hyperglycemia experiments were conducted on the same day and time as the control littermates undergoing circulatory arrest at 20°C for 1.75 h. The 20°C temperature and 1.75-h arrest interval were chosen, because our previous investigations have characterized the presence and distribution of brain damage using these two variables during circulatory arrest in newborn dogs(13–15).
Statistical analysis of the data were accomplished using analysis of variance with Dunnett correction, single multivariate analysis of variance with either random pair or litter effect, and correlation of coefficients.
Institutional approval. The experiments described here were reviewed by the Animal Care and Use Committee of the Pennsylvania State University College of Medicine-the Milton S. Hershey Medical Center, and most recently approved on October 22, 1993.
RESULTS
General findings. A total of 24 newborn dogs from eight litters were subjected to hypothermic circulatory arrest. To minimize intra- and interlitter variability, the puppies underwent hypothermic circulatory arrest in pairs, one of which received a bolus i.v. injection of glucose and the other 1 N saline. All animals were successfully resuscitated and weaned from muscular paralysis and artificial ventilation within 3-4 h after resuscitation, at which time they were extubated. There was no difference in the duration from resuscitation to weaning between the two groups. The interval from onset of cardiopulmonary resuscitation to the appearance of a spontaneous heart rate ranged from 3 to 21 min and was not significantly different between the groups (hyperglycemia, 7.4 ± 1.6 min; normoglycemia, 9.0 ± 2.0 min). The duration of rewarming required to attain normothermia also was similar in the two groups (hyperglycemia, 81± 6 min; normoglycemia, 97 ± 8 min). Finally, the interval from successful resuscitation to extubation was similar in the two groups(hyperglycemia, 151 ± 21 min; normoglycemia, 143 ± 17 min). No animal exhibited seizure activity after extubation. All 24 puppies survived 8 h of recovery and underwent perfusion-fixation for neuropathologic analysis.
Systemic physiologic variables. Systemic physiologic variables of the newborn dogs are presented in Table 1 andFigures 1 and2. As has been the case in all previous studies(13, 14), hypothermia per se was associated with decreases in MABP and heart rate to 57 and 32% of the normothermic values, respectively (p < 0.001) and there were no differences in the percent changes in the normoglycemic and hyperglycemic animals (Fig. 1). Paco2, calculated HCO3-, and hematocrit were unchanged from control in both groups during hypothermia (Table 1). Pao2 remained above 8.0 kPa both before circulatory arrest and after resuscitation from circulatory arrest. The injection of 50% glucose was associated with a 212% increase in plasma glucose immediately before circulatory arrest(Fig. 2).
During recovery from 1.75 h of hypothermic circulatory arrest. MABP increased to the normothermic value by 15 min after resuscitation and remained within the normal range thereafter (Fig. 1). Heart rate increased more slowly and achieved baseline by 2 h of recovery, at which time all animals had attained a rectal temperature of 37°C. Once again, no differences were seen in the recovery of either MABP or heart rate between the two groups. By 15 min of recovery, pHa was at or near the control value but was significantly decreased in both groups at 1 h, owing to the development of a metabolic acidemia in the early recovery period(Table 1). After correction of the metabolic acidosis with injections of NaHCO3, acid-base status was normal for the remaining 7 h of recovery. Plasma glucose in the glucose-treated animals remained higher than that of saline controls for the entire 8 h of recovery(Fig. 2).
Neuropathologic findings. Neuropathologic examination of the newborn dogs at 8 h of recovery from hypothermic circulatory arrest showed minor degrees of choroid plexus and ventricular hemorrhage. Histologically, there was evidence of brain damage in all animals of the two treatment groups. As previously described(13, 14), tissue injury was focused on the cerebral cortex, caudate nucleus, and amygdaloid nucleus. In the regions of brain damage, the process of ischemic neuronal change was well advanced. Tissue injury took the form of selective necrosis of individual neurons that exhibited eosinophilic cytoplasm and either pyknotic or karyorrhexic nuclei.
Among the other structures, the hippocampus showed no damage except in a single animal of each group that had a few damaged neurons in the subiculum. The putamen showed a mild degree of damage in 4/12 animals in each group, and the claustrum had mild damage in 6/12 hyperglycemic and 4/12 normoglycemic dogs. The thalamus was minimally involved in two hyperglycemic animals, and the damage consisted of a few eosinophilic neurons in the dorsomedial and posterolateral nuclei. No damage was seen in the thalamus of the normoglycemic dogs. In the brainstem, the reticular nuclei were mildly involved in 5/12 hyperglycemic but in only 1/12 normoglycemic dogs (p < 0.05). The damage was located primarily in the midbrain and pons. Cerebral white matter, cerebellar cortex, dentate nucleus, and the first few segments of the cervical spinal cord that were included in the specimens were normal.
Histologic grading of the extent of damage within cerebral cortex, caudate nucleus, and amygdaloid nucleus of the hyperglycemic newborn dogs and normoglycemic, control littermates, grouped as pairs, is shown inFigure 3. Neuronal necrosis within the cerebral cortex was variable, ranging from 7 to 198 damaged neurons/1 mm2 in the normoglycemic animals and from 2 to 304 damaged neurons in the hyperglycemic animals. Nine/twelve hyperglycemic animals showed greater cerebral cortical injury than their normoglycemic litermates. However, given the variability in the severity of damage, there was no statistically significant difference in the severity of injury between the two groups (p = 0.15 by multivariate analysis of variance). In caudate nucleus, 6/12 hyperglycemic animals showed greater damage than their normoglycemic littermate controls with no statistically significant difference in the extent of damage between the two groups (p = 0.12). In amygdaloid nucleus, 9/12 hyperglycemic animals showed greater damage than their normoglycemic controls, and the extent of damage was greater in the hyperglycemic animals (p = 0.018).
The relationship between the extent of brain damage and plasma glucose concentrations was determined by correlation of coefficients(Fig. 4). Two plasma glucose variables were examined; specificially, average glucose, which was the mean of the plasma glucose concentrations immediately before circulatory arrest and at 15 min and 1 and 2 h of recovery; and maximum glucose, which was the highest single plasma glucose value recorded. A positive direct correlation between tissue injury and both the average and maximum blood glucose existed for both the caudate nucleus and the amygdaloid nucleus but not for the cerebral cortex.
Correlation of coefficients analyses of average and maximum plasma glucose concentrations in relation to brain damage. The average glucose concentration for each animal was calculated as the mean of the plasma glucose concentrations immediately before circulatory arrest and at 15 min and 1 and 2 h of recovery. The maximum plasma glucose concentration was the highest single plasma glucose value. The damage score for each analyzed structure is the number of damaged neurons/1 mm2. Symbols represent individual brains.
The relationship between the extent of brain damage and systemic variables in addition to plasma glucose concentrations also was determined by correlation of coefficients analyses. No correlation existed between the extent of injury within any structure of brain and duration of cardiopulmonary resuscitation, duration of rewarming to 37°C, duration of postischemic hypotension, or duration of postischemic metabolic acidosis (data not shown). Also, no correlation existed between the extent of damage within any structure and pHa, Paco2, plasma HCO3-, hematocrit, MABP, or heart rate at any measured interval (see Table 1 and Fig. 1).
DISCUSSION
It is noteworthy that, despite recent publications regarding the short-term neurologic outcome of human infants undergoing hypothermic circulatory arrest, there is little information on the effect of fluctuating blood glucose concentrations on subsequent neurologic development(16–18). Glauser et al.(19) studied 40 newborn and developing infants with hypoplastic left heart syndrome and found a significant association between hyperglycemia (plasma glucose >13.9 mmol/L; 250 mg/dL) and necrosis of the cerebral cortex and between hypoglycemia (plasma glucose <2.2 mmol/L; 40 mg/dL) and either periventricular leukomalacia or brainstem necrosis. Twenty of the 40 infants did not undergo surgical correction of their congenital defect; accordingly, many of the brain lesions seen pathologically were not the consequence of hypothermic circulatory arrest but rather of spontaneously occurring cerebral hypoxiaischemia. It remains to be seen whether or not the alterations in systemic glucose homeostasis were causally related to the hypoxic-ischemic brain damage.
In the present investigation in newborn dogs, hyperglycemia with plasma glucose concentrations ranging from 17 to 36 mmol/L was associated with greater damage to the amygdaloid nucleus and probably the caudate nucleus and brainstem than normoglycemia, although the extent to injury to the cerebral cortex was not affected by the high glucose level. Clearly, hyperglycemia was not beneficial to newborn dog brain undergoing hypothermic circulatory arrest(see below). The reason for a lack of a detrimental effect of glucose on the cerebral cortex is not entirely clear but might relate to the variability in the severity of damage within this structure in our experimental model. Statistical analysis of the data for cerebral cortex indicated that, for a significant difference to be seen, an additional 47 dog pairs (hyperglycemia and normoglycemia) would be required to attain a power of 0.80, an inordinate number of animals.
Although hyperglycemia clearly is deleterious to the adult brain subjected to ischemia, the finding is not universal in immature animals. Glucose supplementation of the 7-d postnatal rat actually protects the brain from hypoxic-ischemic damage(20, 21), and metabolic studies in fetal and newborn sheep and newborn dogs suggest a similar protective influence of glucose on the perinatal brain subjected to hypoxia-ischemia(22–25). However, hyperglycemia has been shown to increase ischemic brain damage at normothermia in newborn pigs. In this regard, LeBlanc et al.(26) subjected 0-3 d postnatal piglets to bilateral carotid artery ligation combined with systemic hypoxia and hypotension. Before or during the hypoxic-ischemic insult, the animals were rendered either hyperglycemic with 50% glucose or mildly hypoglycemic with insulin. Neuropathologic examination revealed greater brain damage in the glucose-supplemented piglets than in the insulin-treated controls or in normoglycemic animals derived from prior investigations. Regions with greater tissue injury included cerebral cortex, hippocampus, and basal ganglia, vulnerable structures in this experimental animal model.
The proposed mechanism whereby hyperglycemia accentuates ischemic brain damage, at least in adult animals, relates to an excessive production of tissue lactic acid or to an associated derangement in pH homeostasis(27–29). Presumably, excessive lactate production during hyperglycemic cerebral ischemia is caused by a greater acceleration of anaerobic glycolytic flux than that which occurs when the circulating glucose concentration is not increased. We have not conducted metabolic studies in our newborn dog model of hypothermic circulatory arrest to ascertain rates of cerebral glucose utilization or lactate production in ischemia sensitive and insensitive regions of brain.
Another plausible, albeit less likely, mechanism of accentuated ischemic tissue injury in the hyperglycemic newborn dogs relates to alterations in plasma (and brain) osmolality produced by the i.v. injection of 50% glucose. Although plasma osmolality measurements were not conducted in the present experiments, plasma hyperosmolality would be expected, with osmolalities exceeding those of the normoglycemic animals by up to 13 mOs/L before, during, and for the first 2 h of recovery. Because glucose enters even the newborn brain by facilitative diffusion(30), tissue hyperosmolality likely also occurred in the hyperglycemic animals, especially in the pre- and postischemic intervals, when anaerobic glycolysis would be minimal or nonexistant. The tissue hyperosmolality, in turn, would contribute to cerebral edema and the ultimate neuronal necrosis in the vulnerable structures. To dissociate the metabolic and osmolar effects of glucose on the ischemic brain would require a separate investigation, comparing glucose to a nonmetabolizable sugar, e.g. O-methylglucose.
The variability in the severity of damage especially to the cerebral cortex in both the hyperglycemic and normoglycemic newborn dogs merits discussion. We have no explanation for the observed variability other than the knowledge that it appears unrelated to any of several systemic physiologic and biochemical variables; including those described in the last paragraph of“Results.” We have previously demonstrated that the duration of hypothermic circulatory arrest is an important determinant of the extent of tissue injury, i.e. the longer the interval of ischemia, the greater the brain damage(13, 15): in the present investigation, all animals underwent circulatory arrest for precisely 1.75 h. The most likely explanation for the variability in extent of damage within the cerebral cortex relates to minor differences in the temperature of this structure during and after the ischemic process. Although rectal temperature was maintained within a narrow range (20 ± 0.5°C) in the present investigation, it is plausible that brain temperature fluctuated more widely within any individual animal. It is presently well known that minor variations in body and brain temperature (1-3°C) influence dramatically the severity of hypoxic-ischemic or ischemic brain damage in both adult and immature animals(31–33).
Thus, the available data-including those presented here-indicate a complex interplay between glucose and perinatal hypoxic-ischemic brain damage. Under situations of focal or global partial cerebral ischemia at normothermia, as seen in the immature rat undergoing carotid artery ligation or the perinatal lamb subjected to asphyxia, glucose appears to have a beneficial effect on cerebral metabolic recovery and neuropathologic outcome(20–25). Under conditions of severe or total cerebral ischemia, as seen in the newborn pig model of brain damage at normothermia, glucose is deleterious. For the newborn dog undergoing hypothermic circulatory arrest, glucose supplementation and its associated hyperglycemia is certainly not protective and appears to be deleterious, at least to selected structures of the brain. Possibly, hypothermia per se offers protection to the perinatal brain not only by prolonging the interval required for damage to occur but also by minimizing any potential deleterious effect of hyperglycemia, especially to cerebral cortex. Whether or not hyperglycemia accentuates ischemic brain damage during cardiac arrest at normothermia in the newborn dog presently is undergoing clarification in our laboratory.
Abbreviations
- MABP:
-
mean arterial blood pressure
- Paco2:
-
partial pressure of arterial CO2
- Pao2:
-
partial pressure of arterial O2
- pHa:
-
arterial pH
References
Ferry PC 1987 Neurologic sequelae of cardiac surgery in children. Am J Dis Child 141: 309–312
Greeley WJ, Ungerleider RM, Smith LR, Reeves JG 1989 The effects of deep hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral blood flow in infants and children. Thorac Cardiovasc Surg 97: 737–745
Sealy WC 1989 Hypothermia: its possible role in cardiac surgery. Ann Thorac Surg 47: 788–791
Stevenson JG, Stone EF, Dillard DH 1974 Intellectual development of children subjected to prolonged circulatory arrest during hypothermic open heart surgery in infancy. Circulation 49: 54–59
Dickinson DF, Sambrooks JE 1979 Intellectual performance in children after circulatory arrest with profound hypothermia in infancy. Arch Dis Child 54: 1–6
Wells FC, Coghill S, Caplan HL 1983 Duration of circulatory arrest does influence the psychological development of children after cardiac operations in early life. J Thorac Cardiovasc Surg 86: 823–831
Brunberg JA, Reilly EL, Doty DB 1974 Central nervous system consequences in infants of cardiac surgery using deep hypothermia and circulatory arrest. J Cardiovasc Surg 49: 60–68
Myers RE, Yamaguchi S 1977 Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch Neurol 34: 65–74
Pulsinelli WA, Waldman S, Rawlinson D, Plum F 1982 Hyperglycemia converts neuronal damage into brain infarction. Neurology 32: 1239–1246
Kalimo H, Rehncrona S, Soderfeldt B, Olsson Y, Siesjo BK 1981 Brain lactic acidosis and ischemic cell damage. 2. Histopathology. J Cereb Blood Flow Metab 1: 313–327
Pulsinelli WA, Levy DE, Sigsbee B, Scherer P, Plum F 1983 Increased damage after ischemic stroke in patients with hyperglycemia with or without diabetes mellitus. Am J Med 74: 540–544
Palmer C, Vannucci RC, Christensen MA, Brucklacher RM 1989 Regional cerebral blood flow and glucose utilization during hypothermia in newborn dogs. Anesthesiology 71: 730–737
Mujsce DJ, Towfighi J, Yager JY, Vannucci RC 1993 Neuropathologic aspects of hypothermic circulatory arrest in newborn dogs. Acta Neuropathol 85: 190–198
Mujsce DJ, Towfighi J, Heitjan DF, Vannucci RC 1994 Differences in intra-ischemic temperature influenced neurological outcome after deep hypothermic circulatory arrest in newborn dogs. Stroke 25: 1433–1442
Mujsce DJ, Towfighi J, Vannucci RC 1990 Physiologic and neuropathologic aspects of hypothermic circulatory arrest in newborn dogs. Pediatr Res 28: 354–360
Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KCK, Farrell DM, Holmes GL, Helmers SL, Constantinou J, Carrazana E, Barlow JK, Walsh AZ, Lucius KC, Share JC, Wessel DL, Hanley FL, Mayer JE, Castaneda AR, Ware JH 1993 A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med 329: 1057–1064
Miller G, Rodichok LD, Baylen BG, Myers JL 1994 EEG changes during open heart surgery on infants aged 6 months or less: relationship to early neurologic morbidity. Pediatr Neurol 10: 124–130
Rogers BT, Msall ME, Buck GM, Lyon NR, Norris MK, Roland J-MA, Gingell RL, Clevland DC, Pieroni DR 1995 Neurodevelopmental outcome of infants with hypoplastic left heart syndrome. J Pediatr 126: 496–498
Glauser TA, Rorke LB, Weinberg PM, Clancy RR 1990 Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics 85: 991–1000
Vannucci RC, Mujsce DJ 1992 Effect of glucose on perinatal hypoxic-ischemic brain damage. Biol Neonate 62: 215–224
Hattori H, Wasterlain CG 1990 Posthypoxic glucose supplementation reduces hypoxic-ischemic brain damage in the neonatal rat. Ann Neurol 28: 122–128
Hope PL, Cady EB, Delpy DT, Ives NK, Gardiner RM, Reynolds EOR 1988 Brain metabolism and intracellular pH during ischemia: Effects of systemic glucose and bicarbonate administration studied by31 P and 1H nuclear magnetic resonance spectroscopy in vivo in the lamb. J Neurochem 50: 1394–1402
Chao CR, Hohimer AR, Vissonnette JM 1989 The effect of elevated blood glucose on the electroencephalogram and cerebral metabolism during short-term brain ischemia in fetal sheep. Am J Obstet Gynecol 161: 221–228
Rosenberg AA, Murdaugh E 1990 The effect of blood glucose concentration on postasphyxia cerebral hemodynamics in newborn lambs. Pediatr Res 27: 454–459
Young RSK, Petroff OAC, Aquila WJ, Chueng A, Gore JC 1991 Hyperglycemia and the rate of lactic acid accumulation during cerebral ischemia in developing animals: in vivo proton MRS study. Biol Neonate 59: 136–142
LeBlanc MH, Huang M, Vig V, Patel D, Smith EE 1993 Glucose affects the severity of hypoxic-ischemic brain injury in newborn pigs. Stroke 24: 1055–1062
Rehncrona S, Rosen I, Siesjo BK 1980 Excessive cellular acidosis: an important mechanism of neuronal damage in the brain?. Acta Physiol Scand 110: 435–437
Welsh FA, Ginsberg MD, Reider W, Budd WW 1980 Deleterious effect of glucose pretreatment on recovery from diffuse cerebral ischemia in the eat. II. Regional metabolic levels. Stroke 11: 355–363
Pulsinelli WA, French J, Rawlinson D, Plum F 1982 Cerebral ischemia damages neurons despite lowered brain lactate levels. Ann Neurol 12: 86
Cremer JE 1982 Substrate utilization and brain development. J Cereb Blood Flow Metab 2: 394–407
Busto R, Dietrich WD, Globus MY-T, Valdes I, Schenberg P, Ginsberg MD 1987 Small differences in intra-ischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 7: 729–738
Ridenour TR, Warner DS, Todd MM, McAllister AC 1992 Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 23: 733–738
Yager J, Towfighi J, Vannucci RC 1993 Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 34: 525–529
Acknowledgements
The authors thank the following individuals for their excellent technical assistance: Robert M. Brucklacher, B.S., Cathy M. Housman, B.S., and Lisa Seaman, B.S.
Author information
Authors and Affiliations
Additional information
Supported by the National Institute of Child Health and Human Development Grant P01 HD30704.
Rights and permissions
About this article
Cite this article
Vannucci, R., Rossini, A. & Towfighi, J. Effect of Hyperglycemia on Ischemic Brain Damage during Hypothermic Circulatory Arrest in Newborn Dogs. Pediatr Res 40, 177–184 (1996). https://doi.org/10.1203/00006450-199608000-00001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199608000-00001
This article is cited by
-
Give me less sugar: how to manage glucose levels in post-anoxic injury?
Intensive Care Medicine (2014)
-
Emergency Preservation and Resuscitation with Profound Hypothermia, Oxygen, and Glucose Allows Reliable Neurological Recovery after 3 h of Cardiac Arrest from Rapid Exsanguination in Dogs
Journal of Cerebral Blood Flow & Metabolism (2008)