Abstract
Although cerebral hemorrhage is a widely occurring neurologic disorder thought to be caused by fluctuating blood flow, the response to flow in the neonatal cerebrovasculature has not been characterized. In the present study, we examined the effect of changing flow on middle cerebral artery diameter and pathways by which flow modulates cerebrovascular tone. Arteries from 2-14-d-old piglets were mounted on cannulas and bathed in and perfused with physiologic saline solution. An electronic system controlled pressure and a syringe pump provided constant flow. The transmural pressure was held constant at 20 mm Hg, and changes in vessel diameter were measured as flow was increased in steps from 0 to 1.60 mL/min (flow/diameter curves). Increasing flow at constant pressure resulted in constriction at flows from 0.077 to 0.152 mL/min and dilation at flows from 0.212 to 1.60 mL/min. The flow/diameter curves were repeated in arteries bathed in Na+-reduced or Ca2+-free physiologic saline solution; denervated with 6-hydroxydopamine; or treated with indomethacin, N-nitro-L-arginine methyl ester, Nω-nitro-L-arginine (NLA), and L-arginine), ryanodine, or glutaraldehyde. In Na+-reduced and in Ca2+-free physiologic saline solution, flow constriction was eliminated. Neither indomethacin nor 6-hydroxydopamine affected the biphasic response. N-Nitro-L-argininel, NLA, and ryanodine blocked dilation, whereas L-arginine restored dilation in NLA-treated arteries. These data suggest that neither prostaglandins nor adrenergic nerve endings participate in flow-induced responses in piglet cerebral arteries. Elimination of flow-constriction by Na+ reduction or Ca2+ removal is consistent with findings in other artery types. The elimination of dilation byN-nitro-L-arginine methyl ester, NLA, and ryanodine suggests that dilation is mediated by nitric oxide and intracellular Ca2+. Whereas the contractile and dilatory responses to agonists remained intact after glutaraldehyde perfusion, both flow-induced constriction and dilation were eliminated, indicating that both types of flow responses result from endothelial cell deformation.
Similar content being viewed by others
Main
Fluctuations in cerebral blood flow and/or arterial pressure in the neonate can result in cerebrovascular hemorrhage(1–3), and possibly significant morbidity or mortality(4). The mechanisms that control cerebral blood flow, particularly in the neonate, are still not fully defined. Despite this, there are very few studies characterizing the response of cerebral arteries to changes in flow, and all appear to have been done in adult animals. For example, in vivo, adult rat cerebral arteries exhibited flow-induced dilation that did not appear to result from prostaglandin or nitric oxide (NO) production(5). In adult rabbit cerebral arteries, Garcia-Roldan and Bevan described endothelium-independent responses that were altered by changing transmural pressure(6, 7) and thus levels of arterial tone. At low intravascular pressure the arteries dilated to flow, but at high intravascular pressures they constricted.
The cerebrovasculature of the neonate differs in several important respects from the adult. Neonatal arteries have thinner vascular walls than vessels from adult animals(8) and thus may have an underdeveloped ability to regulate flow. This may partially explain the findings that cerebral arteries from neonatal piglets(9) and dogs(10) had a reduced autoregulatory range that increased with maturation. In addition, piglet cerebral arteries have a significantly depolarized membrane potential compared with adult arteries(9) and consequently may be hypersensitive to mechanical stimuli. It has been found that a change in the membrane potential in smooth muscle cells from rabbit cerebral arteries altered their response to flow(11). Based upon all of these findings, we speculated that the response of cerebral arteries from a newborn animal to changes in flow and pressure might differ from those previously described in adult arteries.
We examined the effect of changing luminal flow on cerebral arteries from neonatal piglets using an electromechanical system designed in our laboratory to control pressure and flow independently in an isolated, cannulated artery. Reports in the literature vary as to whether flow-induced responses are dependent upon an intact endothelial cell layer, and whether NO(12), prostaglandins(13), and ionic currents(14) are involved. Therefore, we investigated possible mechanisms by which flow may modulate cerebrovascular tone in neonatal arteries by pharmacologically blocking the production of NO and prostaglandins, varying extracellular sodium (Na+) and calcium(Ca2+) concentrations, denervating the arteries, and decreasing endothelial cell deformability.
METHODS
Vessel Preparation
This study was approved by the Animal Care and Use Committee at the Zablocki VA Medical Center.
Neonatal pigs (2-14 d old; 1.5-4.0 kg) were premedicated with xylazine (2-3 mg/kg) and ketamine (12-16 mg/kg) and then anesthetized with intraperitoneal sodium pentobarbital (25-30 mg/kg). The animals were exsanguinated and the brain removed. Middle and anterior cerebral arteries were carefully dissected and kept in cold (4°C) PSS until use. The composition of the PSS (in mM) was: 141 Na+, 4.7 K+, 2.5 Ca2+, 0.72 Mg2+, 124 Cl-, 1.7 H2PO-4, 22.5 HCO-3, and 11 glucose.
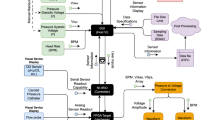
A diagram of the system used to study cannulated blood vessels is shown in Figure 1. The system consists of a waterjacketed plastic chamber in which proximal (inflow) and distal (outflow) cannulas are mounted. The cannulas are glass micropipettes made with a pipette puller (Brown-Flaming P-77, Sutter Instrument Co., Novato, CA) and modified to assure that both tip diameters are equal.
System to control pressure and flow. The syringe pump provides constant flow. The pressure controller uses the signals from the pressure transducers to electronically control the motion of the micromanipulator, increasing or decreasing outflow resistance. The micropipette inserted into the lumen of the artery permits recording of actual luminal pressure. The pulse damper removes oscillations in flow from the syringe pump. Inflow, outflow, and mean (luminal) pressures are digitally displayed by the pressure controller.
A 4-8-mm segment of piglet cerebral artery (outside diameter = 492.9± 131.5 μm; n = 75) was tied onto a 200-μm outside diameter tapered glass cannula with a 22-μm nylon suture. The opposite end was likewise threaded onto a cannula so that fluid could flow through the vessel. The exterior of the vessel was suffused with PSS from a water-jacketed reservoir at 37°C and aerated with a gas mixture containing O2, CO2, and N2, giving a Po2 of 130-150 Torr, Pco2 of 37-40 Torr, and pH of 7.37. The vessel was filled with PSS aereated with the same gas mixture as the reservoir, and all side branches were tied off. Gas concentrations and pH were measured with a blood gas analyzer (model 278, Corning Inc., Corning, NY). A micrometer connected to the proximal cannula was turned to set the artery at its approximate in vivo resting length. The artery was pressurized at 20 mm Hg, and it was allowed to stabilize for 60-90 min in the absence of flow before further study.
Flow Control System
Figure 1 shows the system designed in our laboratory to control pressure and flow independently in an isolated, cannulated artery. A syringe pump (Harvard model 976) connected to the inflow cannula could be set to a constant flow. A pulse damper reduced oscillations in flow caused by slippage of the syringe pump. An electronically driven micromanipulator (Oriel A18008 Encoder Mike, Oriel Corporation, Stratford, CT) was incorporated into a specially designed feedback control circuit. A steel bar attached to the micromanipulator positioned above the outflow tubing was electronically raised or lowered to open or constrict the tubing lumen, which changed the outflow resistance and thereby the pressure. The motion of the micromanipulator was determined by the difference between the pressure measured by the inflow and outflow pressure transducers and a desired pressure set by the user. Thus, pressure and flow were independently controlled, because the syringe pump provided constant flow independent of pressure changes, and the micromanipulator controlled the vessel pressure based on the desired and the measured pressures and was independent of flow changes.
To verify that the pressure measured by the transducers and displayed by the system as the mean value of the inflow and outflow pressures equaled the actual luminal pressure, the luminal pressure was recorded independently with a micropuncture measurement system (Instruments for Physiology and Medicine model 5A, San Diego, CA). A beveled-tip micropipette (5-10-μm diameter) was introduced through the artery wall, and the transmural pressure was recorded. Under constant flow conditions the luminal pressure was changed in 10-mm Hg steps from 0 to 60 mm Hg, and the value recorded by the micropuncture system was compared with the pressure displayed by the pressure controller. Pressure was then held constant while flow was increased from 0.077 to 1.60 mL/min, and transmural pressure was measured.
Vessel Diameter Measurements
A stereomicroscope (Olympus SZ-STB1) situated over the vessel chamber supported a color video camera (Panasonic Digital 5000) which projected the vessel image on a video monitor (Sony PVM-1390). External diameters of the vessel were measured using a video scaler (FORA IV-550) accurate to±1.5 μm. In measuring external diameter we were particularly careful to keep the artery in focus and to measure vessel dimensions at the same point on the wall as judged by the presence of various distinguishing features located near the site (i.e. adhering connective tissue and side branches). The video image of the vessel could also be recorded on a video cassette recorder (Panasonic AG-1730) for later analysis or review. Vessel diameters were measured immediately after mounting the artery, after preconditioning and throughout the protocols described below.
Experimental Protocols
All arteries were tested for viability by measuring the constriction induced by 30 mM potassium chloride (KCl). The vessels were tested for a functionally intact endothelium by adding 10-6 M NE followed by 10-6 M acetylcholine (ACh) at the peak of the NE-induced constriction. Arteries that did not contract by at least 20% to KCl and/or dilate to ACh were discarded.
F/D curves. Initial experiments were performed at transmural pressures of 20 and 60 mm Hg, corresponding to the lower and upper ends of the piglet autoregulatory range(9), to determine whether the level of transmural pressure affected the arterial responses to changing flow. Luminal flow was increased from 0 to 0.077, 0.108, 0.152, 0.212, 0.297, 0.416, 0.583, 0.816, 1.14, and 1.60 mL/min. This range of flows was chosen based upon values reported in the literature for human infants(15, 16). Flow was maintained for 3 min after each step change at which time the external diameter measurement was stable.
The intraluminal pressure of the piglet cerebral vessels can range between 35 and 45 mm Hg, approximately 65-70% of the normal mean systemic pressure of 55-65 mm Hg. However, in vivo, the intracranial pressure produced by the cerebrospinal fluid and the surrounding tissue results in an actual transmural pressure that can range between 25 and 40 mm Hg. Because we wished to avoid undue damage to, or stress on, the vessels due to transient increases in pressure when flow was changed, the rest of the protocols were performed with transmural pressure at 20 mm Hg.
To ensure that the responses obtained in the previous protocol were flow-dependent rather than time-dependent, some arteries were exposed first to high flow (1.60 mL/min) and the diameter was measured at 3 min. Flow was stopped, and the artery allowed to equilibrate for 15 min and then low flow(0.077 mL/min) was turned on, and the diameter was measured. In addition, to determine whether the level of existing tone altered the response to flow, some arteries were first preconstricted with 30 mM KCl and then exposed to low flow (0.077 mL/min) followed by high flow (1.60 mL/min).
Extracellular Na+. Because flow-induced responses in other artery types have been shown to depend on extracellular Na+(14, 17), sucrose was substituted equiosmotically for Na+ in the PSS(18). The Na+ concentration was reduced by 80% based on protocols used in previous studies(5, 14) and also by 10 and 20% to correspond to the daily changes and extremes that can be encountered in infants(17). These concentrations also allowed us to determine whether there was a threshold Na+ concentration at which the response to flow was altered. Thirty minutes after exposure to the Na+-reduced PSS, flow was increased in steps from 0 to 1.60 mL/min, and diameter changes were recorded as described above. To assure that the change in response to decreased Na+ was in fact due to a change in Na+ concentration, and not to a change in charge distribution, the F/D curve was repeated with 80% of the Na+ substituted with lithium chloride(LiCl).
Extracellular Ca2+. In some arteries, the exterior and the luminal PSS were replaced with a Ca2+-free solution containing (in mM): 117 Na+, 4.7 K+, 117 Cl-, 21.2 Mg2+, 24 HCO-3, 1 H2PO-4, 1.17 SO-4, and 5.5 glucose. After 30 min in Ca2+-free PSS, the flow experiments were repeated. F/D curves were also performed in treating the arteries with RYN (10-6 M) to deplete intracellular Ca2+ stores(19).
Adrenergic innervation. To determine whether adrenergic nerve played a role in the flow-induced responses, the adrenergic nerve endings of some arteries were destroyed with 6-OHDA (10-5 M)(20). The completeness of denervation was verified by fluorescence histochemistry(21).
Prostaglandins. Prostaglandins have been shown to play a role in flow-induced dilation in skeletal(13) and femoral(22) arteries. Therefore, some of the arteries were treated with IND at a concentration (10-6 M) that has been shown to block the prostaglandin production in adult arteries(23).
NO. Other arteries were treated with either L-NAME (10-3 M) or NLA (10-3 M) to inhibit NO release(12, 24). Inhibition was verified by the lack of dilation to ACh after NE-induced constriction. In the NLA-treated arteries, the F/D curves were performed in the presence of NLA alone and again after adding L-ARG(10-2 M) to restore NO production.
Endothelial cell deformation. When flow increases, shear stress increases proportionally. It has been suggested(25–27) that deformation of the vascular endothelial cell layer is the catalyst for production of endothelial factors which might contribute to flow induced responses. To determine whether endothelial deformation was involved in the biphasic response to flow in the piglet cerebral arteries, we perfused some vessels with a 0.025% glutaraldehyde solution for 30 s. This method has been shown to reduce endothelial cell deformability but leave the vasodilatory response to ACh intact(26). To ensure that smooth muscle cell integrity and endothelial cell chemical reactivity were still intact after GLU perfusion, the arteries were retested with KCl and ACh before repeating the F/D curves.
Statistical Analysis
All diameter measurements are expressed as percent original diameter(diameter at zero flow under control conditions; mean ± SEM). Comparisons of diameters before and after interventions was made byt test (paired and unpaired as applicable). A value of p< 0.05 was considered statistically significant.
RESULTS
Seventy-five cerebral arteries were used in this study. During the preconditioning period, the arteries developed spontaneous tone and at the end of the period their diameters averaged 93.8 ± 1.40% of their diameters at mounting. The average contractile response to KCl was 38.6 ± 3.50%.In response to NE, artery diameter decreased 16.4 ± 2.80%. When ACh was applied at the peak of the NE-induced contraction, the arteries dilated to 97.0 ± 1.80% of their preconstricted diameter, thus demonstrating the presence of a functional endothelium. The spontaneous tone and the responses to KCl, NE, and ACh were statistically significant(p < 0.05).
Effect of changing transmural pressure and flow. During flow experiments, the transmural pressure value displayed on the controller was within ± 1 mm Hg of the luminal pressure recorded with the micropuncture system. During flow changes, pressure as measured on the controller and with the micropipette, fluctuated approximately ± 2 mm Hg immediately after the change in flow and stabilized within 10.0 ± 0.4 s.
The arteries exhibited a biphasic response to flow (n = 43; Fig. 2). When flow was initiated at constant pressure, artery diameter decreased steadily at flows from 0.077 to 0.212 mL/min, with a maximum decrease of 10.1 ± 1.8% occurring at 0.212 mL/min. As flow was increased in steps from 0.212 mL/min to the maximum of 1.60 mL/min, the arteries dilated, returning to 99.1 ± 3.6% of starting diameter. The flow response was not pressure-dependent because there were no significant differences between responses at 20 and those at 60 mm Hg (n = 4; Fig. 3). Shear stresses at these flow rates ranged from 0.70 to 28 dynes/cm2 as calculated by the formula, t = 4μQ/πr3, where μ is viscosity in poise,Q is flow in mL/s, and r is artery radius in cm.
F/D curves performed in arteries with transmural pressures of 20 mm Hg (n = 43). Increasing flow in steps from 0 to 1.60 mL/min resulted in a biphasic response; constriction at low flows (0.077 to 0.212 mL/min) and dilation at high flows (0.297 to 1.60 mL/min). *Diameter significantly decreased from diameter at zero flow (p < 0.05; paired t test); †diameter significantly greater than at 0.212 mL/min (flow rate where greatest constriction occurred; p < 0.05; paired t test).
When arteries were exposed immediately to the highest flow (1.60 mL/min), they dilated, and when they were then exposed to the lowest flow (0.077 mL/min), they constricted (n = 5; Fig. 4A). When 30 mM KCl was added to the bathing solution to increase vessel tone, the arteries still constricted at low flow and dilated at high flow (n = 4; Fig. 4B).
(A) Average percent change in external diameter when arteries were exposed to high flow, allowed to rest and then exposed to low flow (n = 5; *p < 0.05 compared with zero flow; one sample t test). (B) Average percent change in external diameter in response to low and high flow in arteries whose basal tone was increased by adding 30 mM KCl (n = 4; *p < 0.05 compared with control; one sample t test).
Extracellular Na+. When the Na+ content of the PSS was reduced by 80%, the artery diameter decreased by 23.8 ± 2.4% at zero flow (n = 5; p < 0.05; Fig. 5A). When flow was turned on to 0.077 mL/min, the diameter immediately increased by 15.6 ± 7.2%. As flow increased, the vessel diameter continued to increase, reaching a final value almost 20% higher than in normal PSS (p < 0.05).
(A-D) F/D curves performed in arteries before and after reducing the Na+ content of the PSS: (A) 80% reduction, (B) 20% reduction, (C) 10% reduction,(D) 80% LiCl substitution (n = 5 for each panel; transmural pressure = 20 mm Hg). *Diameter significantly different from diameter at same flow under control conditions; †diameter significantly different from diameter at zero flow under the same conditions (p< 0.05; A-C, paired t test; D, unpairedt test).
Replacing 20% of the Na+ had no effect on the starting diameter of the vessels (n = 5; Fig. 5B). When flow was turned on and increased, flow-induced constriction was absent and the arteries dilated to increased flow similar to the responses seen when Na+ was reduced by 80%. A 10% reduction in Na+ also had no significant effect on starting artery diameter (n = 5; Fig. 5C). However, when flow was turned on, the arteries exhibited a biphasic response to flow similar to the control vessels.
When LiCl was substituted for 80% of the Na+, starting artery diameter at zero flow was reduced by 18.6 ± 4.5% (p < 0.05). When flow was turned on and increased, the arteries dilated to all levels of flow (n = 5; Fig. 5D). The response to low flow in the Li-substituted arteries was similar to that obtained in arteries with the 80% sucrose substitution, suggesting that it was the change in extracellular Na+ concentration and not a change in charge distribution that was responsible for the eliminating flow-induced constriction. In all of the Na+-substitution experiments, returning the vessels to control PSS restored the response to control levels.
Extracellular Ca2+ In Ca2+-free solution, arterial diameter increased 12.3 ± 5.5% above the diameter in Ca2+-containing solution (p < 0.05; n = 4; Fig. 6A). There was no evidence of flow-induced constriction between 0.077 and 0.152 mL/min. As flow was increased to 1.60 mL/min, vessel diameter increased slightly but not significantly. Restoring Ca2+ restored the original response to flow. Depleting intracellular Ca2+ stores with RYN did not change the vessel starting diameter (n = 3; Fig. 6B). When flow was turned on and increased the arteries constricted between 0.077 and 0.212 mL/min. However, the flow-induced dilation seen under control conditions at flows greater than 0.212 mL/min was reversed, and arteries constricted as flow increased from 0.212 to 1.6 mL/min.
Adrenergic denervation and indomethacin treatment. The responses of 6-OHDA-treated arteries were not significantly different from those observed under control conditions (p > 0.05; n = 4, Fig. 7A). Treatment with IND resulted in a slight, but not significant decrease in starting artery diameter at zero flow (p> 0.05; n = 4; Fig. 7B). When flow was turned on and increased, the response to flow was similar to that seen under control conditions.
Chemical blockade of NO. Arteries exposed to either L-NAME or NLA had a significant decrease in starting diameter (p < 0.05). Treatment with L-NAME resulted in flow-induced constriction at all flows up to 0.816 mL/min, whereupon the vessel diameter plateaued at approximately 25% below the control diameter (n = 5; Fig. 8A). When flow was turned on after NLA treatment, arterial diameter remained relatively constant at low flows, approximately 23% below the control diameter, and did not dilate at flows above 0.152 mL/min (n = 4; Fig. 8B). At zero flow, adding L-ARG to NLA-treated arteries resulted in a significant increase in starting diameter (p< 0.05; n = 4; Fig. 8C). When flow was turned on, the arteries dilated at flows from 0.077 to 0.297, and remained dilated as flow was increased.
F/D curves after treatment with (A) L-NAME(10-3 M; n = 5); (B) NLA (10-3 M;n = 4); and (C) after adding LL-ARG (10-2 M) to NLA-treated arteries (n = 4). Transmural pressure = 20 mm Hg.*Diameter significantly different from diameter at the same flow under control conditions; †diameter significantly different from diameter at zero flow under the same condition (p < 0.05; unpaired t test for A, paired t test for B andC).
Decreasing endothelial cell deformability. After the arteries were perfused with GLU, they still retained the ability to constrict to KCl and dilate to ACh and their responses were not significantly different from those seen under control conditions (n = 5; Fig. 9). However, when flow was turned on and increased, both the flow-induced constriction and the dilation were absent and the arterial diameter remained constant over the entire range of flows (n = 5; Fig. 10).
DISCUSSION
In this study we used a system constructed in our laboratory to control pressure and flow accurately and independently in an isolated, cannulated cerebral artery. This system differs from those previously described(6, 14, 28) in that pressure and flow are controlled independently, and the system operates electronically and does not require manual adjustments. The system responded quickly to changes in pressure due to changes in flow or reference pressure and stabilized at the desired pressure within seconds.
Using this system, we describe for the first time the response to flow in an isolated cerebral artery from a neonatal animal. We found that intraluminal flow in isolated piglet cerebral arteries produced a biphasic response at flows similar to those seen in newborn humans(15, 16). The vessels constricted at low flows (between 0.77 and 0.212 mL/min) and dilated at higher flows (0.297 to 1.60 mL/min). The dilation at high flow was not simply a time-dependent phenomenon, because reversing the sequence of flow exposure still resulted in dilation at high flows and constriction at low flows. The responses to flow did not depend on the level of transmural pressure or agonist-induced tone, nor did they appear to be mediated by adrenergic nerve stimulation or prostacyclin release. Flow-induced constriction did depend on the extracellular concentration of both Na+ and Ca2+, whereas flow-induced dilation appeared to be mediated by NO. Both constrictor and dilator responses appeared to be a result of endothelial cell deformation due to shear stress.
In adult rabbit cerebral arteries, Garcia-Roldan and Bevan(6, 7) found that the level of transmural pressure affected the responses to flow. At a flow of 20 μL/min and a transmural pressure of 30 mm Hg, the arteries dilated(6). When pressure was increased to 60 mm Hg at the same flow rate, the arteries dilated only slightly. However, when pressure was raised to 90 mm Hg, the vessels constricted. In a different study, Garcia-Roldan and Bevan(7) found that arteries at 60 mm Hg did not respond to a flow of 20 μL/min, but constricted when flow was increased to 100μL/min. If the transmural pressure was increased to 90 mm Hg, flow-induced constriction was observed at both 20 and 100 μL/min. In our study, the neonatal cerebral arteries exhibited similar biphasic responses to flow at both 20 and 60 mm Hg pressures. Because the response to flow was the same at both pressures, the flow-induced response in the neonatal cerebral arteries does not appear to depend on the level of pressure or tone as is the case in adult rabbit arteries. Rather, our finding is consistent with those of Hoogerwerf et al.(29, 30), who showed that flow-induced constriction in rabbit femoral arteries was not altered by the level of transmural pressure(29) nor by NE-induced preconstriction(30). Kuo et al.(28) also found that pig coronary arteries exhibited flow-induced dilation over a large pressure range.
Garcia-Roldan and Bevan(7) speculated that the change in response to flow with increasing pressure in adult rabbit cerebral arteries was due to a change in the myogenic tone of the vessel, because under no-flow conditions, arterial diameter decreased when pressure increased. In piglet cerebral arteries, increasing pressure in the absence of flow resulted in a maintained but not decreased diameter(9); therefore, there may not be a strong myogenic component influencing the flow responses in these vessels. Although species differences may account for some of the discrepancies between our results and those of Garcia-Roldan and Bevan, it also seems highly likely that there are maturational differences in the ability of the cerebral circulation to regulate pressure and flow.
In the present study, adrenergic nerves did not appear to contribute to flow-induced responses in the piglet cerebral arteries, because denervation with 6-OHDA did not significantly alter the arterial responses to flow. This is in contrast to the finding by Bevan et al.(31) that denervated ear arteries from 4-wk-old rabbits showed increased contraction and decreased dilation to flow. Again, this discrepancy may be due to differences in species and vascular bed as well as to the differences in experimental methodology used.
Although prostaglandins, particularly prostacyclin, have been shown to mediate flow-induced dilation in the femoral(22) and skeletal(13) vascular beds, they did not appear to be active in the flow-induced responses in piglet cerebral arteries. Treating the arteries with IND resulted in a smaller arterial diameter at zero flow, perhaps due to blockade of basal prostacyclin production, but there was no significant effect on the contractile or dilatory response to flow. This corresponds with results obtained from studies in the rat cerebral circulation which showed that flow-induced dilation that was not affected by indomethacin administration(5). Furthermore, both flow-induced constriction and dilation in adult rabbit cerebral arteries were still present after endothelium removal(7, 32), suggesting that prostaglandin production by the endothelium was not responsible for either response.
Flow-induced dilation in the piglet cerebral arteries appeared to be mediated by endothelial cell NO production. Treating the arteries with L-NAME or NLA to inhibit NO release resulted in a significant decrease in starting artery diameter at zero flow, indicating a possible blockade of basal NO production. With both inhibitors, the flow-induced dilation was abolished, suggesting that the flow dilation in the piglet cerebral arteries is NO-dependent. When L-ARG was added to NLA-treated arteries, the diameter increased significantly at zero flow, indicating NO production. When flow was then turned on, flow constriction was not observed, and the arteries dilated to flow. A possible explanation for the absence of the flow-induced constriction may be that the availability of excess L-ARG resulted in increased NO production which overrode the flow constriction seen under control conditions. These results contradict the finding in adult rat cerebral arteries that inhibiting NO production byN-nitro-monomethyl-L-arginine did not alter the response to flow(5). However, in various other vascular beds, flow-induced dilation has been shown to depend upon NO production(13, 24, 33, 34), and the shear stresses calculated for the flows used in our studies were well within the range of values that have been shown to result in NO release(35). Moreover, increasing endothelial cell rigidity abolished flow-induced dilation in the piglet cerebral arteries, indicating that perturbation of the endothelial cell surface is necessary for the response to flow. This is consistent with the findings by Kuchan and Frangos(25) in cultured endothelial cells, and Lamontagneet al.(33) in rabbit coronary arteries, that increased levels of shear stress resulted in increased production of NO.
Flow-induced dilation was also absent when the piglet cerebral arteries were treated with RYN to deplete intracellular Ca2+ stores. This finding further supports the possibility that NO is responsible for the flow-induced dilation because NO synthase, the enzyme that converts L-ARG to NO, is thought to be regulated by intracellular Ca2+(25). Flow-induced dilation was also decreased in Ca2+-free PSS. This may indicate that entry of extracellular Ca2+ contributes to flow-induced dilation, perhaps by increasing the level of free intracellular Ca2+. However, it does not unequivocally mean that flow-induced dilation depends on extracellular Ca2+ because the arteries may have been maximally dilated and simply unable to respond further.
The biphasic response to flow observed in the piglet cerebral arteries is intriguing because at a constant pressure, most investigators have reported either a constrictor(7, 29, 30, 36, 37) or a dilator(5, 12, 13, 24, 28, 32, 34, 38, 39) response to flow, but not both. Although endothelium-dependent flow-induced dilation has been documented in numerous studies(13, 24, 28, 34, 38, 39), flow-induced constriction has typically been described as an endothelium-independent phenomenon(7, 30, 37). This contrasts with our finding wherein decreasing endothelial cell deformability eliminated constriction at low flows. Furthermore, the flow-induced constriction at low flows was dependent upon the extracellular Na+ concentration, a finding consistent with data showing that Na+-dependent, flow-induced responses in rabbit ear resistance arteries were blocked by amiloride, a Na+ channel blocker(14). As Na+ levels may affect vascular tone through a variety of mechanisms, including Na+/H+ exchange, Na+/Ca2+ exchange, and stretch-activated channels(40), further studies are necessary to elucidate the nature of the Na+-dependent pathway in flow-induced constriction in the piglet cerebral arteries.
Flow-induced dilation has been proposed as a mechanism to augment metabolically induced vasodilation (i.e. reactive hyperemia). Although the increase in flow relieves the metabolic demand, it could conceivably result in damage due to stretching of the capillary walls as volume increased. In the adult animal, the increase in flow might be compensated for by other regulatory mechanisms (i.e. myogenic autoregulation, arteriolar autoregulation); in the neonate such regulatory mechanisms appear to be underdeveloped or absent. Flow-induced constriction may serve as a protective mechanism in the neonate. Rather than allowing the increased flow to reach the arterioles and capillaries and cause damage as described above, the arteries would instead constrict and either maintain or reduce the flow to the capillary bed.
Although it is unclear which response(s) occurs in vivo in the adult animal, our finding that intraluminal flow resulted in a biphasic response, constriction at low flows and dilation at high flows, may be significant in the pathogenesis of cerebral hemorrhage in the neonate. Changes in flow within the range that we used experimentally are commonly seen in newborns(15, 16). If, as our data indicate, increasing flow produces cerebrovasodilation, it is possible that, when small arterioles and capillaries are exposed to fluctuating arterial flows and pressure, vascular damage and/or rupture could occur.
Abbreviations
- 6-OHDA:
-
6-hydroxydopamine
- ACh:
-
acetylcholine
- F/D:
-
flow/diameter
- GLU:
-
glutaraldehyde
- IND:
-
indomethacin
- L-ARG:
-
L-arginine
- L-NAME:
-
N-nitro-L-arginine methyl ester
- NE:
-
norepinephrine
- NLA:
-
Nω-nitro-L-arginine
- NO:
-
nitric oxide
- PSS:
-
physiologic saline solution
- RYN:
-
ryanodine
References
Volpe JJ 1979 Cerebral blood flow in the newborn infant: relation to hypoxic-ischemic brain injury and perivascular hemorrhage J. Pediatr 94: 170–173.
Volpe JJ 1981 Current concepts in neonatal medicine. N Engl J Med 304: 886–890.
Volpe JJ 1989 Intraventricular hemorrhage and brain injury in the premature infant. Clin Perinatal 16: 361–410.
Fanaroff AA, Martin RJ 1987 Neonatal-Perinatal Medicine. CV Mosby, St. Louis, pp 520–528.
Fujii K, Heistad DD, Faraci FM 1991 Flow-mediated dilation of the basilar artery in vivo. Circ Res 69: 697–705.
Garcia-Roldan JL, Bevan JA 1990 Flow-induced constriction and dilation of cerebral resistance arteries. Circ Res 66: 1445–1448.
Garcia-Roldan JL, Bevan JA 1991 Augmentation of endothelium-independent flow constriction in pial arteries at high intravascular pressures. Hypertension 17: 870–874.
Pearce WJ, Longo LD 1991 Developmental aspects of endothelial function. Semin Perinatol 15: 40–48.
Norins NA, Madden JA 1991 Developmental changes in cerebral artery autoregulation in piglets. FASEB J 5:A674.
Hernandez MJ, Brennan RW, Bowman GS 1980 Autoregulation of cerebral blood flow in the newborn dog. Brain Res 184: 199–202.
Bevan JA, Wellman GC 1993 Intraluminal flow-initiated hyperpolarization and depolarization shift the membrane potential of arterial smooth muscle toward an intermediate level. Circ Res 73: 1188–1192.
Cooke JP, Stamler, Andon N, Davies PF, McKinley G, Loscalzo J 1990 Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol 259:H804–H812.
Koller A, Messina EJ, Wolin MS, Kaley G 1989 Endothelial impairment inhibits prostaglandin and EDRF-mediated arteriolar dilationin vivo. Am J Physiol 257:H1966–H1970.
Bevan JA, Joyce EH 1992 Comparable sensitivity of flow contraction and relaxation to Na reduction may reflect flow-sensor characteristics. Am J Physiol 263:H182–H187.
Cowan F, Whitelaw A 1991 Acute effects of acetazolamide on cerebral blood flow velocity and pCO2 in the newborn infant. Acta Paediatr Scand 80: 22–27.
Raju TNK, Kim SY 1989 Cerebral artery flow velocity acceleration and deceleration characteristics in newborn infants. Pediatr Res 26: 588–592.
Bevan JA, Siegel G 1991 Blood vessel wall matrix flow sensor: evidence and speculation. Blood Vessels 28: 552–556.
Hermsmeyer K, Harder DR 1986 Membrane ATPase mechanism of K+ return relaxation in arterial muscles of stroke-prone SHR and WKY rats. Am J Physiol 250:C557–C562.
Vadula MS, Kleinman JG, Madden JA 1993 Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol 265:L591–L597.
Aprigliano O, Hermsmeyer K 1976 In vitro denervation of the portal vein and cerebral artery of the cat. J Pharmacol Exp Ther 198: 568–577.
Dela Torre JC, Surgeon JW 1976 A methodological approach to rapid and sensitive monoamine histofluoresence using a modified glyoxcylic acid technique: the SPG method. Histochemistry 49: 81–93.
Hecker M, Mulsch A, Bassenge E, Busse R 1993 Vasoconstriction and increased flow: two principal mechanisms of shear stress-dependent endothelial autocoid release. Am J Physiol 265:H828–H833.
Jackson WF 1986 Prostaglandins do not mediate arteriolar oxygen reactivity. Am J Physiol 250:H1102–H1108.
Rubanyi GM, Romero JC, Vanhoutte PM 1986 Flow-induced release of endothelium-derived relaxing factor. Am J Physiol 250:H1145–H1149.
Kuchan MJ, Frangos JA 1994 Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol 266:C628–C636.
Mel'kumyants AM, Balashov TA, Smishko V, Khayutin VM 1986 Selective blocking of arterial sensitivity to blood flow rate by glutaraldehyde. Byull Eksp Biol Med 101: 524–526.
Smiesko V, Johnson PC 1993 The arterial lumen is controlled by flow-related shear stress. News Physiol Sci 8: 34–38.
Kuo L, Davis MJ, Chilian WM 1990 Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol 259:H1063–H1070.
Hoogerwerf N, van der Linden PJW, Sipkema P 1989 Effects of oxygen and flow on the diameter of the femoral artery of the rabbit. Blood Vessels 26: 360–367.
Hoogerwerf N, Zijlsta EJ, vander Linden PJW, Westerhof N, Sipkema P 1992 Endothelium function is protected by albumin and flow-induced constriction is independent of endothelium and tone in isolated rabbit femoral artery. J Vasc Res 29: 367–375.
Bevan RD, Clementson A, Joyce E, Bevan JA 1993 Sympathetic denervation of resistance arteries increases contraction and decreases relaxation to flow. Am J Physiol 264:H490–H494.
Bevan JA, Joyce EH, Wellman GC 1988 Flow-dependent dilation in a resistance artery still occurs after endothelium removal. Circ Res 63: 980–985.
Lamontagne D, Pohl U, Busse R 1992 Mechanical deformation of vessel wall and shear stress determine the basal release of endothelium-derived relaxing factor in the intact rabbit coronary vascular bed. Circ Res 70: 123–130.
Pohl U, Herlan K, Huang A, Bassenge E 1991 EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol 261:H2016–H2023.
Olesen SP, Clapham DE, Davies PF 1988 Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature 331: 168–170.
Henrion D, Laher I, Bevan JA 1992 Intraluminal flow increases vascular tone and Ca2+ influx in the rabbit facial vein. Circ Res 71: 339–345.
Sipkema P, van der Linden PJW, Hoogerwerf N, Westerhof N 1989 Does the endothelium play a role in flow-dependent constriction?. Blood Vessels 26: 368–376.
Koller A, Kaley G 1990 Role of endothelium in reactive dilation of skeletal muscle arterioles. Am J Physiol 259:H1313–H1316.
Pohl U, Dezsi L, Simon B, Busse R 1987 Selective inhibition of endothelium-dependent dilation in resistance-sized vesselsin vivo. Am J Physiol 253:H234–H239.
Lansman JB, Hallam TJ, Rink TJ 1987 Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers?. Nature 325: 811–813.
Acknowledgements
The authors thank Drs. J. H. Linehan and L. D. Nelin for their helpful comments and P. A. Keller for his technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by Department of Veterans Affairs Medical Research Service funds awarded to J.A.M. and by the Department of Pediatrics, The Medical College of Wisconsin and Marquette University. L.A.S. is the recipient of a Wisconsin Heart Association Predoctoral Fellowship.
Rights and permissions
About this article
Cite this article
Shimoda, L., Norins, N., Jeutter, D. et al. Flow-Induced Responses in Piglet Isolated Cerebral Arteries. Pediatr Res 39, 574–583 (1996). https://doi.org/10.1203/00006450-199604000-00002
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199604000-00002
This article is cited by
-
Isolated Human and Rat Cerebral Arteries Constrict to Increases in Flow: Role of 20-HETE and TP Receptors
Journal of Cerebral Blood Flow & Metabolism (2011)