Abstract
Background:
The burden of chronic obstructive pulmonary disease (COPD) is high. Health benefits can be gained in primary care by early detection and preventive measures.
Aims:
To compare the effectiveness of two strategies for population-based early detection of COPD, taking into account different socioeconomic status (SES) settings.
Methods:
Practices were randomised on strategy and stratified on SES setting. The Respiratory Health Screening Questionnaire (RHSQ) was distributed to all participants. In the practice-managed condition, the practice was responsible for the whole procedure, while in the patient-managed condition, patients were responsible for calculating their RHSQ risk score and applying for a spirometry test. The main outcome measure was the rate of COPD diagnoses after screening.
Results:
More new COPD patients were detected in the practice-managed condition (36%) than in the patient-managed condition (18%). In low SES practices, more high-risk patients were found (16%) than in moderate-to-high SES practices (9%). Recalculated for a standard Dutch practice (2,350 patients), the yield would be 8.9 new COPD diagnoses, which is a 20% increase of known cases.
Conclusions:
The practice-managed variant of this screening procedure shows a substantial yield of new COPD diagnoses for both low and moderate-to-high SES practices.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent causes of morbidity and mortality worldwide. It represents the largest fraction of mortality for respiratory diseases, which form the third most common cause of death in the European Union (8%).1–5 The worldwide prevalence of COPD in the general population is rising steeply to 10% among those aged 40 years or more.6–8 The prevalence of detected COPD in Dutch general practices is 2%.9 In many western countries, another 2% of COPD remains undiagnosed.10
Miravitlles et al. found that only 60% of people with chronic respiratory symptoms consulted a physician and only 45% of them underwent spirometry.11 Our research showed that high-risk patients detected by screening reported having had respiratory symptoms previously without seeking help.12 Smokers are particularly unaware of having symptoms and neglecting to see a doctor. They feel shame and guilt because of a self-inflicted disease associated with persistent smoking habits13 and they adapt to slowly developing respiratory problems.14
Cigarette smoking is the main causal factor of COPD in the western world and is also the most important risk factor that can be influenced.15,16 Starting age, total pack-years, and current smoking status are all predictors of COPD mortality.17 Definitive smoking cessation makes sense at any stage of the disease and slows the decline of forced expiratory volume in one second (FEV1) from 60mL/year to 30mL/year.18 Even temporary cessation helps to slow the decline.19 The success of smoking cessation is greater when people participate in a prevention programme that includes spirometry.20
Low socioeconomic status (SES) is also an important risk factor for COPD. It correlates significantly with lower lung function, even after adjustment for smoking status, occupational exposures, and race or ethnic origin.21 The impact of low SES on lung function is variable, but FEV1 reductions of >300mL in men and >200mL in women have been reported.22 Non-smokers with low SES can be seen as independent groups at risk of COPD.23
A third risk factor for COPD — independent of SES and smoking — is poor health literacy. It is associated with more severe COPD stages, greater COPD helplessness, worse respiratory-related quality of life, and more use of COPD-related emergency healthcare.24
Early detection programmes combined with smoking cessation interventions may delay the progression of COPD.25 The literature reveals several COPD case-finding initiatives among patients in waiting rooms, patients with co-morbidities,26–31 and among smokers,32–34 but only a few population-based screening studies of COPD.12,35–37 All these studies report 13–41% additional COPD diagnoses.
The Respiratory Health Screening Questionnaire (RHSQ) is a population-based questionnaire for early COPD detection in a random population, and it includes a validated set of questions and a score card system (Table 1).38,39 The RHSQ was piloted in a Dutch general practice population in 2008 and found 11% of the respondents to be at high risk. After post-bronchodilator spirometry, 39.6% of these high-risk respondents were diagnosed with COPD.12 These results prompted us to evaluate this combination of questionnaire and spirometry on a larger scale. Anticipating a possible nationwide rollout of the RHSQ, we had to take into account the consequences of extra workload for family practices. A standard Dutch practice has 2,350 patients, including 700 in the target group for the RHSQ (age 40–70 years). It was estimated that conducting the RHSQ would take about 10 hours per standard practice (half for preparation, half for settlement). We therefore tested two strategies: one in which the practice is responsible for the whole procedure and another in which the practice is responsible for only the recruitment and the respondent is responsible for handling the RHSQ and requesting a spirometric test when indicated.
The aim of this study was to determine the yield of a population-based early detection procedure including (a) risk screening by the RHSQ and (b) spirometric testing of high-risk patients in primary care. Our research questions were:
-
How many new COPD diagnoses are found by this procedure?
-
Is there a difference between a practice-managed and a patient-managed strategy?
-
How does the SES profile of the practice influence the effectiveness of these strategies?
Methods
Setting and design
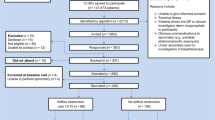
We conducted a cluster randomised controlled trial among 16 family practices of different sizes from May to September 2012. The practices were located in four cities in the middle and south of the Netherlands. The RHSQ was sent to all eligible patients registered with these practices. In the patient-managed condition, patients were responsible for calculating their RHSQ scores and for demanding a spirometry test in case of a high-risk score. In the practice-managed condition the family practice was responsible for the whole process.
Practices were stratified based on socioeconomic status (SES). There is no universally accepted definition of SES, and stratification criteria vary from occupation, educational level, income, and residential area.40 We took the criterion of residential area based on the Dutch Health Authority public register. In this register practices are classified as low SES or moderate to high SES depending on the socioeconomic profile of their adhering area (based on postal codes).
Population
People aged 40–70 years, excluding those already diagnosed with asthma or COPD or with other serious lung diseases such as lung cancer, pneumoconiosis, tuberculosis, bronchiectasis, and pneumonectomy, were eligible to take part in the study. People using oxygen supplementation and those with impaired mobility were also excluded. Ethical approval was obtained from Medical Ethics Review Board (MERB), Atrium Hospital, Heerlen (MERB number 12N33).
Intervention
The RHSQ is a validated questionnaire for screening patients at risk of COPD (Table 1).38,39 It was posted to patients and responders had the opportunity of mailing or emailing by log-in code. It contains 10 simple questions about age, smoking history, body weight, body length, and respiratory complaints (Table 1). The standard version includes a scoring card to calculate the risk of COPD: low risk (<16.5 points), medium risk (16.5–19.5 points), or high risk (>19.5 points). People in the patient-managed condition used this scoring card to calculate their risk themselves; the scoring card advised them to consult the family practitioner in case of a high-risk score. In the practice-managed condition the scoring card was removed and risks were calculated by the family practice; people with a high-risk score were explicitly invited for a spirometry test.
The spirometry test was conducted according to the prevailing guidelines of the Dutch College of General Practitioners.41 A COPD diagnosis was based on the combination of a post-bronchodilator FEV1/FVC ratio <0.7 and the physician's clinical evaluation.42 All participants gave informed consent.
Outcome measures
The main outcome measure was the number of new COPD diagnoses after spirometry per practice. Secondary outcome measures were the rate of RHSQ high-risk scores and the yield of new COPD diagnoses in a standard Dutch practice (2,350 patients). Determinants were the intervention strategy, the SES profile of the practice, the smoking status of the patient, and the practice prevalence of COPD prior to the screening.
Data sampling and analysis
Data from questionnaires and spirometry tests were collected in a central database and completed with interview data about smoking status. To get insight into the reasons for non-response, a sample of 10 non-responders per practice were approached by telephone with one open question — their reason for non-participation.
Descriptive and testing statistics were calculated by SPSS-19. Differences between groups were assessed by χ2 tests after correction for cluster randomisation and stratification by logistic regression. Correlation between prior prevalence and percentage of newly detected COPD was evaluated by Pearson's correlation test.
Results
Response and demographics
The enrolled practices included 11,498 patients aged 40–70 years. A total of 1,390 patients (597 with a known COPD diagnosis) met one or more of the exclusion criteria. The RHSQ was distributed in the enrolled practices to 10,108 people and a total of 3,573 responded. The response to the questionnaire was 50% in the practice-managed condition (52% low SES, 47% moderate to high SES) and 27% in the patient-managed condition (24% low SES, 29% moderate to high SES). Table 2 shows that both conditions were similar with respect to age and gender, but not for smoking behaviour. The patient-managed condition yielded fewer smokers, which suggests a response bias on smoking behaviour in favour of non-smokers. Table 3 shows that respondents in low SES practices were on average older, more often current smokers, and had more pack-years than respondents in moderate to high SES practices.
The attendance for spirometry among high-risk respondents was 54% in the practice-managed condition (49% low SES, 64% moderate to high SES) and 75% in the patient-managed condition (78% low SES, 73% moderate to high SES). Reasons for non-participation in low SES practices were lack of time, other health problems, no understanding, and no risk awareness while, in moderate to high SES practices, reasons for non-participation were no complaints, forgotten, lack of time, and no interest.
Test outcomes
Table 4 shows that there was no significant difference in the rate of high-risk scores between respondents in the two conditions, which does not reveal evidence for a response bias based on the risk test score. In the practice-managed condition, however, high-risk respondents had more COPD than in the patient-managed condition (36% vs. 18%, p<0.05). This difference was even larger among active smokers (54% vs. 25%).
Low SES practices had significantly more high-risk respondents than moderate to high SES practices (16% vs. 9%, p<0.05). However, there was no significant difference between the two groups with respect to the chance of having COPD.
On the practice level, there was no correlation between the prior prevalence of COPD and the percentage of newly detected COPD diagnoses (Pearson's coefficient −0.167).
The yield of our screening procedure for early detection was 73 new COPD diagnoses out of 10,108 participants for both strategies, giving an average of 8.9 new COPD diagnoses per standard Dutch practice in the practice-managed condition and 3.0 in the patient-managed condition. Thus, the practice-managed approach was approximately three times as effective as the patient-managed approach.
Cost per detected case
Table 5 shows that, for a standard Dutch practice, 77 RHSQs had to be distributed (61 low SES, 111 moderate to high SES), three spirometry tests had to be performed (in both SES settings) and, additionally, one COPD consultation had to be undertaken to detect one new COPD diagnosis in the practice-managed condition. The total cost of the detection programme was 荤256 per detected case (荤224 low SES, 荤324 moderate to high SES). In the patient-managed condition, 256 RHSQs had to be distributed and six spirometry tests had to be performed, with a cost of 荤698 per detected case.
Discussion
Main findings
Our study has shown that the use of the RHSQ among all those aged 40–70 years followed by spirometry testing of high-risk scoring respondents is effective and can best be provided following a practice-managed strategy. A greater responsibility for the patient in this procedure appeared less effective, especially for smokers. We found a return of 8.9 newly detected COPD cases per standard primary care practice, which is an increase of 20% over the known prevalence. An investment of 荤224–324 per detected case seems reasonable for a disease where health benefits can be gained with early detection, since the earlier COPD is detected and behaviour changes are induced, the slower the decline in FEV1 and the better the quality of life prognosis will be,22 especially among smokers18 and low SES groups.43
Interpretation of findings in relation to previously published work
Most newly detected COPD patients in our study were active smokers. This indicates room for improvement, because smoking cessation is still the most effective way to reduce the progression of COPD and to improve survival in COPD patients. Some studies have shown that smokers who know that they have COPD are more successful quitters,20,44 although this observation is not consistent in the literature.
The RHSQ has previously been used only for targeted screening studies among active smokers: Kotz et al.33 found 41% COPD after screening, Price et al.34 found 19%, and Freeman et al.32 reported 17%. To our knowledge, this is the first study where the RHSQ has been used for population-based screening. In its most effective condition (practice-managed), the yield of COPD after screening was 36% among high-risk respondents. This is more than population-based screening studies using different questionnaires: Van Schayck et al.37 reported 18% COPD after screening and Calverley et al.35 reported 13%. Only Martinez et al.36 reported a higher yield of COPD after screening (38%), but their study recruited also from specialist sources.
The possibility that the RHSQ would be less effective in low SES practices because of an expected lower response is not supported by our results. There was no response bias; the RHSQ returned more high-risk persons and more COPD patients after screening in low SES practices than in moderate to high SES practices. The latter finding is in line with the literature, which reports more COPD among low SES groups.21
The assumption that a higher prior prevalence of COPD in a specific practice would leave less room for the detection of new COPD patients is not supported by our study. Furthermore, the apparent underdiagnosis of COPD is illustrated by the relatively low proportion of mild COPD in the Netherlands; of all known COPD cases, 28% have mild disease, 54% have moderate disease, 15% have severe disease, and 3% have very severe COPD.45 This raises the question of how the potential of undetected COPD can be estimated. The Dutch College of General Practitioners (NHG) estimates the prevalence of known COPD cases at 20/1,000, which means that a standard family practice (2,350) has 47 cases of COPD.41 Soriano et al. estimate that there is another 2% of undetected COPD patients, which makes the potential prevalence of known and unknown COPD cases 4%.10 In our most effective strategy (practice-managed) we detected an average of 8.9 new COPD patients per standard Dutch practice. Together with the 47 cases already known, this amounts to 56 COPD cases per family practice, which is 2.4% of the total population, far less than the 4% estimated by Soriano et al. This makes it worthwhile to improve further the response of our early detection procedure.
Strengths and limitations of this study
Although the 50% response to the questionnaire is acceptable, the 54% response to the invitation for spirometry is rather low and needs improvement. One way to improve the response is to conduct the intervention in another season. Our study period included the summer holidays, a time when many people are on vacation, especially in low SES practices when many ethnic Mediterranean people leave for a long visit to their native country. Many candidates for the RHQS may have missed the invitation.
A practical limitation of our study is the fact that the whole intervention was performed under time pressure with the help of external practice nurses and supplementary administrative support. For the personnel of a primary care practice it is impossible to screen and test all patients aged 40–70 years within a period of three months. Without external practice nurse support, it would be more feasible to spread out the effort over a longer time — for example, 15 or 30 months with every month one- or two-year cohorts. We performed a feasibility study for this implementation strategy in 10 other practices and report the results of this study in this same issue of the PCRJ.46
Implications for future research, policy and practice
Future research should focus on the implementation of early diagnosis and treatment of COPD in general practice. Especially important is a full cost-effectiveness study investigating early detection of COPD in combination with smoking cessation.
Conclusions
A population-based COPD screening procedure among those aged ≥40 years with the RHSQ and spirometry is an effective method for the early detection of COPD in primary care. The yield is higher in low SES practices than in moderate to high SES practices. Regardless of SES, this procedure should preferably be managed by the family practice and not by the patients themselves.
References
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL . Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747–57. http://dx.doi.org/10.1016/S0140-6736(06)68770-9
Lopez AD, Shibuya K, Rao C, et al. The global burden of COPD: future COPD projections. Eur Respir J 2006;27:397–412. http://dx.doi.org/10.1183/09031936.06.00025805
Mathers CD, Roncar D . Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:2011–30. http://dx.doi.org/10.1371/journal.pmed.0030442
Murray CJ, Lopez AD . Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498–504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2
Murray CJL, Lopez AD, Black R, et al. Global burden of disease 2005: call for collaborators. Lancet 2007;370:109–10. http://dx.doi.org/10.1016/S0140-6736(07)61064-2
Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741–50. http://dx.doi.org/10.1016/S0140-6736(07)61377-4
Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM . Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523–32. http://dx.doi.org/10.1183/09031936.06.00124605
Menezes AMB, Perez-Padilla R, Jardim JRB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005;366:1875–81. http://dx.doi.org/10.1016/S0140-6736(05)67632-5
van den Berg MJ, Kolthof ED, de Bakker DH, van der Zee J . Tweede Nationale Studie naar ziekten en verrichtingen in de huisartspraktijk — de werkbelasting van huisartsen. [Second National Study to diseases and interventions in primary care — the workload of general practitioners]. Utrecht, The Netherlands: Nivel, 2004.
Soriano JB, Zielinski J, Price D . Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009;374:721–32. http://dx.doi.org/10.1016/S0140-6736(09)61290-3
Miravitlles M, de la Roza C, Morera J, et al. Chronic respiratory symptoms, spirometry and knowledge of COPD among general population. Respir Med 2006;100:1973–80. http://dx.doi.org/10.1016/j.rmed.2006.02.024
Dirven JA, Muris JW, van Schayck CP . COPD screening in general practice using a telephone questionnaire. COPD 2010;7(5):352–9. http://dx.doi.org/10.3109/15412555.2010.510547
Halding A-G, Heggdal K, Wahl A . Experiences of self-blame and stigmatisation for self-infliction among individuals living with COPD. Scand J Caring Sci 2011;25:100–7. http://dx.doi.org/10.1111/j.1471-6712.2010.00796.x
van den Boom G, Rutten-van Molken MP, Tirimanna PR, van Schayck CP, Folgering H, van Weel C . Association between health-related quality of life and consultation for respiratory symptoms: results from the DIMCA programme. Eur Respir J 1998;11(1):67–72. http://dx.doi.org/10.1183/09031936.98.11010067
Bize R, Burnand B, Mueller Y, Rege Walther M, Cornuz J . Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev 2009;(2):CD004705.
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–55. http://dx.doi.org/10.1164/rccm.200703-456SO
Doll R, Peto R, Boreham J, Sutherland I . Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519. http://dx.doi.org/10.1136/bmj.38142.554479.AE
Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS . Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med 2000;161:381–90. http://dx.doi.org/10.1164/ajrccm.161.2.9901044
Murray RP, Anthonisen NR, Connett JE, et al. Effects of multiple attempts to quit smoking and relapses to smoking on pulmonary function. Lung Health Study Research Group. J Clin Epidemiol 1998;51:1317–26. http://dx.doi.org/10.1016/S0895-4356(98)00120-6
Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax 2006;61:869–73. http://dx.doi.org/10.1136/thx.2006.059071
Hegewald MJ, Crapo RO . Socioeconomic status and lung function. Chest 2007;132:1608–14. http://dx.doi.org/10.1378/chest.07-1405
Prescott E, Lange P, Vestbo J . Socioeconomic status, lung function and admission to hospital for COPD: results from the Copenhagen City Heart Study. Eur Respir J 1999;13:1109–14. http://dx.doi.org/10.1034/j.1399-3003.1999.13e28.x
Salvi SS, Barnes PJ . Chronic obstructive pulmonary disease in non-smokers. Lancet 2009;374:733–43. http://dx.doi.org/10.1016/S0140-6736(09)61303-9
Omachi TA, Sarkar U, Yelin EH, Blanc PD, Katz PP . Lower health literacy is associated with poorer health status and outcomes in chronic obstructive pulmonary disease. J Gen Intern Med 2013;28:74–81. http://dx.doi.org/10.1007/s11606-012-2177-3
Van Schayck CP, Loozen JMC, Wagena E, Akkermans RP, Wesseling GJ . Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002;324:1370. http://dx.doi.org/10.1136/bmj.324.7350.1370
Buffels J, Degryse J, Liistro G . Diagnostic certainty, co-morbidity and medication in a primary care population with presumed airway obstruction: the DIDASCO2 study. Prim Care Respir J 2009;18(1):34–40. http://dx.doi.org/10.3132/pcrj.2008.00047
Geijer RMM . Detection of COPD in smokers (Thesis). Utrecht, The Netherlands: Utrecht Medical Center, 2006.
Gingter C, Wilm S, Abholz H-H . Is COPD a rare disease? Prevalence and identification rates in smokers aged 40 years and over within general practice in Germany. Fam Pract 2009;26:3–9. http://dx.doi.org/10.1093/fampra/cmn084
Piperno D, Bart F, Serrier P, Zureik M, Finkielsztejn L . [General practice patients at risk of chronic obstructive pulmonary disease: epidemiologic survey of 3411 patients]. Presse Med 2005;34(21):1617–22. http://dx.doi.org/10.1016/S0755-4982(05)84236-8
Stratelis G, Jakobsson P, Molstad S, Zetterstrom O . Early detection of COPD in primary care: screening by invitation of smokers aged 40 to 55 years. Br J Gen Pract 2004;54:201–06.
Vandevoorde J, Verbanck S, Gijssels L, et al. Early detection of COPD: a case finding study in general practice. Respir Med 2007;101:525–30. http://dx.doi.org/10.1016/j.rmed.2006.06.027
Freeman D, Nordyke RJ, Isonaka S, et al. Questions for COPD diagnostic screening in a primary care setting. Respir Med 2005;99:1311–18. http://dx.doi.org/10.1016/j.rmed.2005.02.037
Kotz D, Nelemans P, van Schayck CP, Wesseling GJ . External validation of a COPD diagnostic questionnaire. Eur Respir J 2008;31:298–303. http://dx.doi.org/10.1183/09031936.00074307
Price DB, Tinkelman DG, Halbert RJ, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration 2006;73:285–95. http://dx.doi.org/10.1159/000090142
Calverley PM, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D . Development of a population-based screening questionnaire for COPD. COPD 2005;2(2):225–32. http://dx.doi.org/10.1081/COPD-57594
Martinez FJ, Raczek AE, Seifer FD, et al. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS). COPD 2008;5(2):85–95. http://dx.doi.org/10.1080/15412550801940721
van Schayck CP, Loozen JM, Wagena E, et al. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002;324:1370. http://dx.doi.org/10.1136/bmj.324.7350.1370
Price DB, Tinkelman DG, Nordyke RJ, Isonaka S, Halbert RJ . Scoring system and clinical application of COPD diagnostic questionnaires. Chest 2006;129:1531–9. http://dx.doi.org/10.1378/chest.129.6.1531
van Schayck CP, Halbert RJ, Nordyke RJ, Isonaka S, Maroni J, Nonikov D . Comparison of existing symptom-based questionnaires for identifying COPD in the general practice setting. Respirology 2005;10:323–33. http://dx.doi.org/10.1111/j.1440-1843.2005.00720.x
Lynch J, Kaplan G . Socioeconomic position. In: Berkman LK, Kawachi I, editors. Social epidemiology. New York: Oxford University Press, 2000: pp13–35.
Smeele IJ, van Weel C, van Schayck CP, et al. NHG-Standaard COPD [NHG Guideline COPD]. Huisarts Wet 2007;50(8):362–79.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of COPD update 2009 (cited Feb 2011). Available from: www.goldcopd.com
Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race, and COPD health outcomes. J Epidemiol Community Health 2011;65(1):26–34. http://dx.doi.org/10.1136/jech.2009.089722
Parkes G, Greenhalgh T, Griffin M, Dent R . Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008;336:598–600. http://dx.doi.org/10.1136/bmj.39503.582396.25
Hoogendoorn M, Rutten-van Molken MPMH, Hoogenveen RT, et al. A dynamic population model of disease progression in COPD. Eur Respir J 2005;26:223–33. http://dx.doi.org/10.1183/09031936.05.00122004
Dirven JAM, Tange JT, Muris JWM, van Haaren KMA, Vink G, van Schayck CP . Early detection of COPD in general practice — implementation, workload and socioeconomic status: an observational study. Prim Care Respir J 2013;22(3):338–43. http://dx.doi.org/10.4104/pcrj.2013.00071
Acknowledgements
Handling editor Anthony D'Urzo
Statistical review Gopal Netuveli
Funding Funding was provided by Lung Foundation Netherlands. Financial compensation of 荤1000 has been offered to the participating general practices.
Author information
Authors and Affiliations
Contributions
Mr Vink of the Lung Foundation chaired the project quality group. The Dutch College of General Practitioners provided input by Dr van Haaren as a member of the quality group. Dr Tange was responsible for the statistical analyses. Dr Muris provided advise as general practitioner and Professor van Schayck was finally responsible for the study and the manuscript. Dr Dirven was the primary investigator and project coordinator. All authors contributed to the text of the manuscript and approved the final manuscript. We thank Mr Jan Hesp from Novivex for contributing external practice nurse and supplementary administrative support.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest in relation to this article. OCPvS is an Assistant editor of the PCRJ, but was not involved in the editorial review of, nor the decision to publish, this article.
Rights and permissions
About this article
Cite this article
Dirven, J., Tange, H., Muris, J. et al. Early detection of COPD in general practice: patient or practice managed? A randomised controlled trial of two strategies in different socioeconomic environments. Prim Care Respir J 22, 331–337 (2013). https://doi.org/10.4104/pcrj.2013.00070
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4104/pcrj.2013.00070
This article is cited by
-
Community-Based Approach to Assess Obstructive Respiratory Diseases and Risk in Urban African American Churches
Journal of Immigrant and Minority Health (2023)
-
Identifying airway obstruction in primary care: is there a role for physiotherapists?
BMC Primary Care (2022)
-
Specialist respiratory outreach: a case-finding initiative for identifying undiagnosed COPD in primary care
npj Primary Care Respiratory Medicine (2021)
-
Point of care microspirometry to facilitate the COPD diagnostic process in primary care: a clustered randomised trial
npj Primary Care Respiratory Medicine (2018)
-
Development and validation of the Salzburg COPD-screening questionnaire (SCSQ): a questionnaire development and validation study
npj Primary Care Respiratory Medicine (2017)