Abstract
Background:
A spirometrically-defined restrictive ventilatory defect is a common finding when performing spirometry.
Aims:
We aimed to determine the frequency, geographical variation, individual consequences, and ‘severity’ of the restrictive ventilatory defect.
Methods:
A population-based study was conducted in Spain. The response rate from 11 participating centres was 88.9%, totalling 3,802 participants. A restrictive ventilatory defect was defined according to pre-bronchodilator spirometry as forced expiratory volume in 1s/forced vital capacity (FEV1/FVC) ≥0.70 and a predicted FVC <80%, in accordance with current American Thoracic Society/European Respiratory Society guidelines.
Results:
The prevalence of a restrictive ventilatory defect was 12.7% (95% CI 9.7% to 15.7%), with the highest in Seville (19.4%) and Burgos (18.5%) and the lowest in Oviedo (5.2%) and Madrid-La Princesa (5.7%) (p<0.001). Although most of the participants (97.1%) with a restrictive ventilatory defect were objectively considered ‘mild’ by spirometry (%predicted FVC 50–80%), they reported more phlegm, dyspnoea, and wheezing than healthy control participants (p<0.001), and scored worse in all St George's Respiratory Questionnaire domains of quality of life and activities of daily living (p<0.001). Interestingly, they scored similarly to participants with chronic obstructive pulmonary disease (COPD) in both (p=0.102 and p=0.217). In a multivariate analysis, older age, male gender, heavy smoking, low education, and high body mass index were independently associated with having a restrictive ventilatory defect.
Conclusions:
A restrictive ventilatory defect in spirometry is a common finding (12.7%) with a highly variable geographical distribution (range 3.7) whose population burden is important in terms of quality of life and activities of daily living and similar to that of an obstructive pattern compatible with COPD.
Similar content being viewed by others
Introduction
A restrictive ventilatory defect is a common finding when conducting spirometry, with a prevalence in the general adult population ranging from 7% to 13% in a number of surveys.1–3 The clinical relevance of a spirometrically-defined restrictive ventilatory defect is uncertain in the absence of respiratory symptoms, signs of pulmonary fibrosis, or other assessments.2 A number of factors have been associated with a restrictive ventilatory defect3 including overweight/obesity, post-tuberculous lung damage,4 ethnic inhalation of tobacco,5 occupational exposures,6,7 vertebral spine and other bone problems,8 and some have even hypothesised an association with the future development of diabetes9 and lung cancer.10 There is growing evidence to suggest that a restrictive spirometric ventilatory defect is relatively common, yet the morbidity and mortality related to this lung function impairment and its relative impact in comparison with the better known obstructive patterns — i.e. chronic obstructive pulmonary disease (COPD) — remains to be fully quantified, both in primary care and at all secondary levels. It is widely recognised that, for an accurate measurement of a restrictive ventilatory defect, it is fundamental to monitor the correct duration and intensity of forced spirometry (>6s in adults and at least 3s in children), as well as fulfilling all standards and quality control set by expert guidelines such as the American Thoracic Society/European Respiratory Society (ATS/ERS) consensus11 and the Primary Care Respiratory Society UK (formerly General Practice Airways Group).12
This study aimed to determine the frequency, severity, geographical variability, and determinants of individual consequences (respiratory symptoms, impact on daily activities and quality of life) of a restrictive ventilatory defect in the population.
Methods
The methodology and protocol of the EPI-SCAN study have been described previously.13 Briefly, EPI-SCAN is a population-based, multicentre, cross-sectional, observational, epidemiological study carried out on a national scale in Spain with a randomised selection of participants using two-stage sampling, stratified by areas close to the participating centres. The following participating centres were selected from four geographical areas of Spain (north, east, south and centre): Barcelona, Burgos, Cordoba, Huesca, Madrid (two centres), Oviedo, Seville, Valencia, Vic, and Vigo. Men and women in the general population aged between 40 and 80 years and resident in Spain were included in the survey.
The fieldwork was performed between May 2006 and July 2007. Information was collected on sociodemographic data, smoking habits, previous diagnosis of respiratory diseases and other pathologies, dyspnoea, and treatment for respiratory diseases, among other variables. The presence of respiratory symptoms (daily morning cough, frequent sputum, and the presence at some time of dyspnoea and wheezing) was collected using the Spanish version of the CECA questionnaire.14 Other questionnaires included the London Chest Activity of Daily Living (LCADL) scale15 translated and validated into Spanish;16 co-morbidities using the Charlson index;17 the generic health status EQ-5D questionnaire18 translated and validated into Spanish;19 and the respiratory-specific health status St George's Respiratory Questionnaire (SGRQ)20 translated and validated into Spanish.21
The study was authorised by the corresponding ethics committees for clinical research (centralised CEI approval at the Hospital Clinic i Provincial de Barcelona CEIC#2006-05-155) and all participants gave their voluntary written consent to participate in the survey.
Spirometry
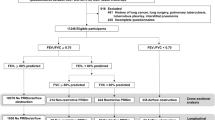
Forced spirometry was carried out with MasterScope CT (VIASY Healthcare®, Hoechberg, Germany) using the acceptability and reproducibility criteria and the selection of manoeuvres proposed in the most recent recommendations of the ATS/ERS;14 the reference values of the ECSC were used.22 The manoeuvres were repeated 15–30min after inhaling 200μg salbutamol. Following the criteria of the ATS/ERS guidelines,23 the bronchodilator test was considered positive if there was an increase in forced expiratory volume in 1s (FEV1) or forced vital capacity (FVC) of >200ml and >12% from the baseline value. The primary focus of EPI-SCAN was chronic obstructive pulmonary disease (COPD), with results to date available elsewhere.24–28 A restrictive ventilatory defect was defined as an FEV1/FVC ≥0.70 and an FVC <80% predicted. By definition and design (Figure 1), an EPI-SCAN participant diagnosed as having COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) cannot have a restrictive ventilatory defect.15
Statistical analysis
The calculation of the sample size of the EPI-SCAN study was carried out assuming a prevalence of COPD of 12%, with an accuracy of ±1% and assuming a 20% drop-out rate.15 Since the mean number of participants in the 11 areas of the EPI-SCAN was 345, with a maximum of 471 in Seville and a minimum of 136 in Oviedo, there is statistical power for most areas with regard to the mean prevalence of both COPD and restrictive ventilatory defect. The results for each variable are shown as the mean with standard deviation in the case of continuous variables, and the number of cases for each category and frequency regarding the total number of responses in the case of categorical variables. The prevalence of restrictive ventilatory defect and its 95% confidence interval was calculated. The statistical significance shown in Appendix 1 and 2 (available online at www.thepcrj.org) was compared in each variable by area in relation to the global EPI-SCAN study, performing first an ANOVA and then a bilateral test for continuous variables and a χ2 test for categorical variables. In the final multivariate analysis the reference categories were: age 40–49 years; female; never smoker; university degree education; Charlson index 0; and normal weight as body mass index (BMI) 18.5–24.9kg/m2. All variables in the bivariate analysis were forced into the multivariate analysis even if they had no significance in the bivariate analysis. Finally, in the adjusted model also by centre, Seville — the centre with the greatest number of participants (n=471) — was considered a posteriori the reference category. A level of statistical significance <0.05 was used in all the statistical tests.
Results
Overall, of 4,274 subjects randomly contacted by telephone at the 11 sites, 3,885 agreed to participate in the study and 3,802 (response rate 88.9%) were available for analysis (complete minimum dataset on gender, age, and lung function). The 389 subjects (9.1%) who refused to take part in the survey were slightly older and included more women and never and former smokers.
From the total population of 3,802 individuals performing full forced spirometry, 52 had pre-bronchodilator (BD) FEV1/FVC ≥0.70, post-BD FEV1/FVC <0.70 and pre-BD FVC <80% and 429 had pre-BD FEV1/FVC ≥0.70, post-BD FEV1/FVC ≥0.70 and pre-BD FVC <80% (marked with an asterisk, Figure 1). The prevalence of a spirometrically-defined restrictive ventilatory defect was therefore 12.7% (95% CI 9.7% to 15.7%) (Table 1), with the highest in Seville (19.4%, 95% CI 11.3% to 27.5%) and Burgos (18.5%, 95% CI 10.0% to 27.0%) and the lowest in Oviedo (5.2%, 95% CI 0 to 21.7%) and Madrid-La Princesa (5.7%, 95% CI 0 to 15.6%), and with a range of 3.7, p=0.000 (Figure 2 and Appendix 1 available online at www.thepcrj.org).
The 481 participants with a restrictive ventilatory defect had a mean age of 63 years, 57% were men, mean BMI 29.3kg/m2 (13.5% with morbid obesity (BMI >35kg/m2)), and had a substantial smoking exposure (mean 31.5 pack-years from 20% current smokers and 33% ex-smokers) (Table 2 and Appendix 2 available online at www.thepcrj.org). Although most (97.1%) of the participants with a restrictive ventilatory defect were objectively considered ‘mild’ by spirometry (%predicted FVC 50–80%), they reported more phlegm, dyspnoea, and wheezing than healthy control participants (p=0.000; Table 2). Compared with patients with COPD, they reported fewer respiratory symptoms but more wheezing (45.5%; p=0.000). Participants with a restrictive ventilatory defect had a significant worsening in mMRC dyspnoea and a higher Charlson index than the reference group but they did not differ from the COPD group (p=0.338; Table 2).
On further assessment, subjects with a restrictive ventilatory defect scored worse in all SGRQ domains of quality of life and in half of the LCADL activities of daily living (self-care and physical, but not domestic and leisure) than the reference group (p=0.000; Table 3). Interestingly, they scored similarly to participants with COPD (p=0.102), except for the utility score of the EQ-5D (p=0.000).
Finally, in a multivariate analysis in the EPI-SCAN participants without COPD, only older age, male gender, heavy smoking (>30 pack-years), low education, and BMI ≥30kg/m2 (even more in those with BMI ≥40kg/m2) were independently associated with having a restrictive ventilatory defect, the last three factors showing a consistent dose-response gradient (Table 4). Both the direction and magnitude of these effects were sustained when additionally adjusted by centre, with an increase in the goodness of fit of the overall model (Cox and Snell r2=0.102 without centre adjustment versus r2=0.126 with centre adjustment).
Discussion
Main findings
The results of this study show that a spirometrically-defined restrictive ventilatory defect is a common spirometric finding (12.7%) which is even more common in the same population than COPD (10.2%).26 It is associated with significant morbidity including a higher frequency of respiratory symptoms and worse outcomes in all SGRQ domains of quality of life and many LCADL activities of daily living than reference participants with ‘normal spirometry’ and comparable morbidity to those with COPD. The study also shows that the population distribution of the restrictive ventilatory defect is geographically highly variable, with a range of 3.7 within the 11 areas surveyed.
Just as the spirometric finding of an obstructive pattern in a smoker at risk of COPD might be considered to be the beginning of a diagnostic process24 eventually leading to a lifestyle and/or therapeutic intervention, we propose that further investigations such as carbon monoxide diffusing capacity and lung volumes may be indicated when a restrictive ventilatory defect is documented after high quality spirometry, even in the absence of other clinical signs and symptoms. At the primary care level, restrictive disease cannot be diagnosed solely by spirometry but can be excluded if vital capacity is normal and, as discussed by Levy et al.,14 a restrictive ventilatory defect finding in primary care requires referral for measurement of total lung capacity and gas transfer in a specialised laboratory. Newly available evidence in the primary care setting has shown that an educational intervention to minimise the risk of low quality spirometries being influenced by poor technique (particularly a poor inspiratory or expiratory effort) might then lead to a reduction in many unnecessary secondary care referrals.25
Strengths and limitations of this study
The EPI-SCAN study of restrictive ventilatory defects has several strengths and limitations. The large sample size, standardised use of tools and spirometry with the highest quality standards, and equiposity among the investigators about the frequency and burden of restrictive ventilatory defect can be confirmed, as it was not intended at the inception of the protocol or fieldwork to study it further. Among the limitations, there were no imaging tests or pathology associated with the study protocol and no further respiratory or cardiovascular tests were performed in these participants. We did not conduct specific quality control tests or further statistical analyses in those participants who were unable to undergo spirometry to prove obstruction. In a recent publication which focused on high-resolution computed tomographic imaging and interstitial lung disease,26 the odds of a restrictive deficit in participants with interstitial abnormalities were 2.3 times the odds in participants without such abnormalities.
Interpretation of findings in relation to previously published work
There is growing evidence for the increased frequency and associated morbidity and mortality of restrictive spirometry. In an Italian study conducted in 265 elderly participants (51.9% men, age 65–97 years), a restrictive ventilatory defect was associated with increased mortality. The authors concluded that the same medical attention should be given to these patients as to those with an obstructive ventilatory defect compatible with COPD. Guerra et al. recently reported a 25-year follow-up study of 2,048 adults; 12% had a restrictive ventilatory defect at baseline which was associated with an increased risk of all-cause mortality (adjusted HR 1.7, 95% CI 1.3 to 2.3) and of life-threatening co-morbidities. Our data add to these reports, documenting worse health status and lower activities of daily living compared with individuals with either ‘normal’ or obstructive spirometry.
Implications for future research, policy and practice
Future studies might further evaluate individuals with a spirometric finding consistent with restriction by profiling the clinical diagnosis given after additional tests and evaluations such as volumes by plethysmography and chest imaging, as well as obesity and other metabolic assessments. More longitudinal data are needed to evaluate further the clinical relevance of a restrictive ventilatory defect.
The reasons for the observed geographical variability (range 3.7) remain largely unknown. Our previous research on the geographical variability of COPD (range 2.7) found no association with COPD mortality or smoking prevalence.27 Further studies of this variation in other populations are therefore needed.
Both the Proyecto Latinoamericano de Investigaciön en Obstrucciön Pulmonar (PLATINO)29 and the Burden of Obstructive Lung Disease (BOLD)30 initiatives have greatly expanded our knowledge of the worldwide distribution of COPD. However, it can be hypothesised that these and other major initiatives that have targeted obstructive spirometry, irrespective of the COPD definition, have perhaps missed an important public health target by not exploring the frequency and outcomes of restrictive spirometry. We therefore encourage a re-analysis of their data to determine the population variability, such as in the study conducted within EPI-SCAN.31 Contrary to COPD underdiagnosis,31 it is largely inappropriate to refer to underdiagnosis with restrictive ventilatory defect. However, we should consider the magnitude of undertreatment with respiratory medications in this study. With 73% of patients with restrictive ventilatory defect not treated with respiratory medications (ranging from 91.3% to 45% in our 11 participating centres; Appendix 2 available online www.thepcrj.org), it can be extrapolated that more than 2.1 million people in Spain have respiratory symptoms that are not managed or clinically identified. From a public health perspective, the combined effects of ageing, obesity, and cumulative smoking at the population level, all reaching epidemic levels — in the developed and also in the developing world — must be emphasised.32 There remain many unknowns regarding the individual and population burden of obstructive and restrictive airflow limitations — from how to define them to how to manage them — both at the primary care level as well as the secondary care level.33,34
Conclusions
We conclude that a spirometrically-defined restrictive ventilatory defect is a common and geographically variable finding, and is associated with an individual burden similar to that of COPD.
References
Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC . Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003;58:388–93. http://dx.doi.org/10.1136/thorax.58.5.388
Ryu JH, Colby TV, Hartman TE . Idiopathic pulmonary fibrosis: current concepts. Mayo Clin Proc 1998;73:1085–101. http://dx.doi.org/10.4065/73.11.1085
Mannino DM, Holguin F, Pavlin BI, et al. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis 2005;9:613e21.
Baig IM, Saeed W, Khalil KF . Post-tuberculous chronic obstructive pulmonary disease. J Coll Physicians Surg Pak 2010;20:542–4.
Ben Saad H, Khemiss M, Bougmiza I, et al. [Spirometric profile of narghile smokers]. Rev Mal Respir 2009;26:299–314. http://dx.doi.org/10.1016/S0761-8425(09)72587-2)
Chakraborty S, Mukherjee S, Roychoudhury S, Siddique S, Lahiri T, Ray MR . Chronic exposures to cholinesterase-inhibiting pesticides adversely affect respiratory health of agricultural workers in India. J Occup Health 2009;51:488–97. http://dx.doi.org/10.1539/joh.L9070
Schenker MB, Stoecklin M, Lee K, et al. Pulmonary function and exercise-associated changes with chronic low-level paraquat exposure. Am J Respir Crit Care Med 2004;170:773–9. http://dx.doi.org/10.1164/rccm.200403-266OC
Payo J, Perez-Grueso FS, Fernandez-Baillo N, Garcia A . Severe restrictive lung disease and vertebral surgery in a pediatric population. Eur Spine J 2009;18:1905–10. http://dx.doi.org/10.1007/s00586-009-1084-8
Ford ES, Mannino DM ; National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Prospective association between lung function and the incidence of diabetes: findings from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Diabetes Care 2004;27:2966–70. http://dx.doi.org/10.2337/diacare.27.12.2966
Purdue MP, Gold L, Jarvholm B, Alavanja MC, Ward MH, Vermeulen R . Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 2007;62:51–6. http://dx.doi.org/10.1136/thx.2006.064196
Miller MR, Hankison J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. http://dx.doi.org/10.1183/09031936.05.00034805
Levy ML, Quanjer PH, Booker R, Cooper BG, Holmes S, Small I . Diagnostic spirometry in primary care: proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations: a General Practice Airways Group (GPIAG) document. Prim Care Respir J 2009;18:130–47. http://dx.doi.org/10.4104/pcrj.2009.00054
Ancochea J, Badiola C, Duran-Tauleria E, et al. [The EPI-SCAN survey to assess the prevalence of chronic obstructive pulmonary disease in Spanish 40-to-80-year-olds: protocol summary.] Estudio EPI-SCAN: resumen del protocolo de un estudio para estimar la prevalencia de EPOC en personas de 40 a 80 años en España. Arch Bronconeumol 2009;45:41–7. http://dx.doi.org/10.1016j.arbres.2008.06.001
Minette A, Aresini G, Sanna-Randaccio F, et al. Cuestionario CECA para el estudio de los síntomas respiratorios, 1987. Luxemburgo 3a ed.: Comisiön de las Comunidades Europeas, 1988.
Garrod R, Bestall JCM, Paul EA, Wedzicha JA, Jones PW . Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London Chest Activity of Daily Living scale (LCADL). Respir Med 2000;94:589–96. http://dx.doi.org/10.1053/rmed.2000.0786
Vilaro J, Gimeno E, Sanchez Férez N, et al. Actividades de la vida diaria en pacientes con enfermedad pulmonar obstructiva crönica: validaciön de la traducciön española y análisis comparativo de 2 cuestionarios. Med Clin (Barc) 2007;129:326–32.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. http://dx.doi.org/10.1016/0021-9681(87)90171-8
The EuroQol Group. EuroQol-5D a new facility for measurement of health-related quality of life. Health Policy 1990;16:199–208. http://dx.doi.org/10.1016/0168-8510(90)90421-9
Badia X, Roset M, Montserrat S, Herdman M, Segura A . La versiön española del EUROQoL: descriptiön y aplicaciones. Med Clin 1999;112(Suppl 1):79–85
Jones PW, Quirk FH, Baveystock CM, Littlejohns P . A self-complete measure of health status for chronic airflow limitation. Am Rev Respir Dis 1992;145:1321–7.
Ferrer M, Alonso J, Prieto L, et al. Validity and reliability of the St George's Respiratory Questionnaire after adaptation to a different language and culture: the Spanish example. Eur Respir J 1996;9:1160–6. http://dx.doi.org/10.1183/09031936.96.09061160
Quanjer Ph, Tammeling G, Cotes J, Pedersen O, Peslin R, Yernault J . Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J 1993;(Suppl 16):5–40.
Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–46. (www.ersnet.org Last updated December 2007).
Soriano JB, Zielinski J, Price D . Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009;374:721–32. http://dx.doi.org/10.1016/S0140-6736(09)61290-3
Carr R, Telford V, Waters G . Impact of an educational intervention on the quality of spirometry performance in a general practice: an audit. Prim Care Respir J 2011;22:210–13. http://dx.doi.org/10.4104/pcrj.2011.00006
Washko GR, Hunninghake GM, Fernandez IE, et al.; COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med 2011;364:897–906. http://dx.doi.org/10.1056/NEJMoa1007285
Scarlata S, Pedone C, Fimognari FL, Bellia V, Forastiere F, Incalzi RA . Restrictive pulmonary dysfunction at spirometry and mortality in the elderly. Respir Med 2008;102:1349–54. http://dx.doi.org/10.1016/j.rmed.2008.02.021
Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD . Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax 2010;65:499–504. http://dx.doi.org/10.1136/thx.2009.126052
Menezes AM, Perez-Padilla R, Jardim JR, et al.; PLATINO Team. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005;366:1875–81. http://dx.doi.org/10.1016/S0140-6736(05)67632-5
Buist AS, McBurnie MA, Vollmer WM, et al.; BOLD Collaborative Research Group.International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741–50. http://dx.doi.org/10.1016/S0140-6736(07)61377-4
Scano G, Innocenti-Bruni G, Stendardi L . Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respir Med 2010;104:925–33. http://dx.doi.org/10.1016/j.rmed.2010.02.019
Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM . The global burden of cancer: priorities for prevention. Carcinogenesis 2010;31:100–10. http://dx.doi.org/10.1093/carcin/bgp263
Varadi RG, Goldstein RS . Pulmonary rehabilitation for restrictive lung diseases. Chest 2010;137:2247–8. http://dx.doi.org/10.1378/chest.09-1857
Culver BH . Obstructive? Restrictive? Or a ventilatory impairment? Chest 2011;140:568–9. http://dx.doi.org/10.1378/chest.11-0935
Acknowledgements
Handling editor Anthony D'Urzo
Statistical review Gopal Netuveli
The authors acknowledge the hard work of all the researchers and collaborators in each centre and thank the participants for their voluntary unselfish collaboration. They also thank Professor David M Mannino, University of Kentucky, for helpful suggestions and advice.
Funding The EPI-SCAN study was sponsored by GlaxoSmithKline S.A., Spain.
Author information
Authors and Affiliations
Contributions
JBS had the original idea and is the guarantor of this study. JBS, FG-R, GS, and JA developed the plan of analysis. DG contributed to the statistical analysis. JBS drafted the report and all co-authors, MM, LM, VS, ED included, contributed to and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
GS is an employee of GlaxoSmithKline S.A. The other authors declare that they have no conflicts of interest in relation to this article.
Appendices
Appendix 1. Demographic and clinical characteristics of EPI-SCAN participants, total and by centres

Appendix 2. Demographic and clinical characteristics of EPI-SCAN participants with spirometrically defined restrictive ventilatory defect, total and by centres

Rights and permissions
About this article
Cite this article
Soriano, J., Miravitlles, M., García-Río, F. et al. Spirometrically-defined restrictive ventilatory defect: population variability and individual determinants. Prim Care Respir J 21, 187–193 (2012). https://doi.org/10.4104/pcrj.2012.00027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4104/pcrj.2012.00027
This article is cited by
-
Longitudinal mortality of preserved ratio impaired spirometry in a middle-aged Asian cohort
BMC Pulmonary Medicine (2023)
-
Reduced forced vital capacity is independently associated with, aging, height and a poor socioeconomic status: a report from the Tunisian population-based BOLD study
BMC Pulmonary Medicine (2022)
-
Restricted spirometry and cardiometabolic comorbidities: results from the international population based BOLD study
Respiratory Research (2022)
-
Reduced forced vital capacity is independently associated with ethnicity, metabolic factors and respiratory symptoms in a Caribbean population: a cross-sectional study
BMC Pulmonary Medicine (2019)
-
Significant predictors of medically diagnosed chronic obstructive pulmonary disease in patients with preserved ratio impaired spirometry: a 3-year cohort study
Respiratory Research (2018)