Abstract
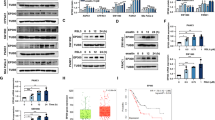
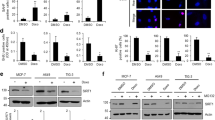
The multispecific transcription factor and tumor suppressor FOXO3 is an important mediator of apoptosis, but the mechanisms that control its proapoptotic function are poorly understood. There has long been evidence that acetylation promotes FOXO3-driven apoptosis and recently a specific JNK (c-Jun N-terminal kinase)-dependent S574 phosphorylated form (p-FOXO3) has been shown to be specifically apoptotic. This study examined whether acetylation and S574 phosphorylation act independently or in concert to regulate the apoptotic function of FOXO3. We observed that both sirtuins 1 and 7 (SIRT1 and SIRT7) are able to deacetylate FOXO3 in vitro and in vivo, and that lipopolysaccharide (LPS) treatment of THP-1 monocytes induced a rapid increase of FOXO3 acetylation, partly by suppression of SIRT1 and SIRT7. Acetylation was required for S574 phosphorylation and cellular apoptosis. Deacetylation of FOXO3 by SIRT activation or SIRT1 or SIRT7 overexpression prevented its S574 phosphorylation and blocked apoptosis in response to LPS. We also found that acetylated FOXO3 preferentially bound JNK1, and a mutant FOXO3 lacking four known acetylation sites (K242, 259, 290 and 569R) abolished JNK1 binding and failed to induce apoptosis. This interplay of acetylation and phosphorylation also regulated cell death in primary human peripheral blood monocytes (PBMs). PBMs isolated from alcoholic hepatitis patients had high expression of SIRT1 and SIRT7 and failed to induce p-FOXO3 and apoptosis in response to LPS. PBMs from healthy controls had lower SIRT1 and SIRT7 and readily formed p-FOXO3 and underwent apoptosis when similarly treated. These results reveal that acetylation is permissive for generation of the apoptotic form of FOXO3 and the activity of SIRT1 and particularly SIRT7 regulate this process in vivo, allowing control of monocyte apoptosis in response to LPS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 2000; 20: 9138–9148.
Tran H, Brunet A, Grenier JM, Datta SR, Fornace Jr AJ, DiStefano PS et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 2002; 296: 530–534.
Chung YW, Kim HK, Kim IY, Yim MB, Chock PB . Dual function of protein kinase C (PKC) in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced manganese superoxide dismutase (MnSOD) expression: activation of CREB and FOXO3a by PKC-alpha phosphorylation and by PKC-mediated inactivation of Akt, respectively. J Biol Chem 2011; 286: 29681–29690.
Hagenbuchner J, Kuznetsov A, Hermann M, Hausott B, Obexer P, Ausserlechner MJ . FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J Cell Sci 2012; 125: 1191–1203.
Ni HM, Du K, You M, Ding WX . Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol 2013; 183: 1815–1825.
Calnan DR, Brunet A . The FoxO code. Oncogene 2008; 27: 2276–2288.
Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 2007; 1: 101–112.
Su JL, Cheng X, Yamaguchi H, Chang YW, Hou CF, Lee DF et al. FOXO3a-dependent mechanism of E1A-induced chemosensitization. Cancer Res 2011; 71: 6878–6887.
Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 2008; 10: 138–148.
Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM, Li PC et al. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res 2008; 10: R21.
Fu Z, Tindall DJ . FOXOs, cancer and regulation of apoptosis. Oncogene 2008; 27: 2312–2319.
Li Z, Zhao J, Tikhanovich I, Kuravi S, Helzberg J, Dorko K et al. Serine 574 phosphorylation alters transcriptional programming of FOXO3 by selectively enhancing apoptotic gene expression. Cell Death Differ 2015; 23: 583–595.
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004; 303: 2011–2015.
North BJ, Verdin E . Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol 2004; 5: 224.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009; 458: 1056–1060.
Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 2013; 18: 416–430.
Tseng AH, Shieh SS, Wang DL . SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 2013; 63: 222–234.
Wang Y, Zhu Y, Xing S, Ma P, Lin D . SIRT5 prevents cigarette smoke extract-induced apoptosis in lung epithelial cells via deacetylation of FOXO3. Cell Stress Chaperones 2015; 20: 805–810.
Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S et al. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol Med 2013; 5: 430–440.
Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med 2007; 39: 8–13.
Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 2010; 285: 33959–33970.
Wang F, Nguyen M, Qin FX, Tong Q . SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 2007; 6: 505–514.
Jin YH, Kim YJ, Kim DW, Baek KH, Kang BY, Yeo CY et al. Sirt2 interacts with 14-3-3 beta/gamma and down-regulates the activity of p53. Biochem Biophys Res Commun 2008; 368: 690–695.
Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO . SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 2010; 123: 4251–4258.
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001; 107: 149–159.
Liu Y, Shepherd EG, Nelin LD . MAPK phosphatases—regulating the immune response. Nat Rev Immunol 2007; 7: 202–212.
Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R et al. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One 2009; 4: e8414.
Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q . Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene 2012; 31: 1546–1557.
Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL . Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem 2001; 276: 38837–38843.
McKeever UM, O'Mahoney C, Lawlor E, Kinsella A, Weir DG, Feighery CF . Monocytosis: a feature of alcoholic liver disease. Lancet 1983; 2: 1492.
Khongkow M, Olmos Y, Gong C, Gomes AR, Monteiro LJ, Yague E et al. SIRT6 modulates paclitaxel and epirubicin resistance and survival in breast cancer. Carcinogenesis 2013; 34: 1476–1486.
Flick F, Luscher B . Regulation of sirtuin function by posttranslational modifications. Front Pharmacol 2012; 3: 29.
Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE et al. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J 2010; 24: 3145–3159.
Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006; 127: 635–648.
Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT et al. Phosphorylation regulates SIRT1 function. PLoS One 2008; 3: e4020.
Zschoernig B, Mahlknecht U . Carboxy-terminal phosphorylation of SIRT1 by protein kinase CK2. Biochem Biophys Res Commun 2009; 381: 372–377.
Gao Z, Zhang J, Kheterpal I, Kennedy N, Davis RJ, Ye J . Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem 2011; 286: 22227–22234.
Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B . Effects of resveratrol in inflammatory arthritis. Inflammation 2007; 30: 1–6.
Lee SM, Yang H, Tartar DM, Gao B, Luo X, Ye SQ et al. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia 2011; 54: 1136–1146.
Lucey MR, Mathurin P, Morgan TR . Alcoholic hepatitis. N Engl J Med 2009; 360: 2758–2769.
Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology 2009; 49: 1326–1334.
Acknowledgements
This study was supported by grant AA012863 from the National Institute on Alcoholism and Alcohol Abuse, a fellowship grant from the Biomedical Research Training Program of the University of Kansas Medical Center (to ZL) and by a grant from the Hubert and Richard Hanlon Trust. The human monocytes used in this study were provided by the University of Kansas Liver Center Tissue Bank. We thank Drs Charles Rice and Robert A Davey for providing reagents for these studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, Z., Bridges, B., Olson, J. et al. The interaction between acetylation and serine-574 phosphorylation regulates the apoptotic function of FOXO3. Oncogene 36, 1887–1898 (2017). https://doi.org/10.1038/onc.2016.359
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.359
This article is cited by
-
The APC/C E3 ligase subunit ANAPC11 mediates FOXO3 protein degradation to promote cell proliferation and lymph node metastasis in urothelial bladder cancer
Cell Death & Disease (2023)
-
Apoptosis evasion via long non-coding RNAs in colorectal cancer
Cancer Cell International (2022)
-
NAD(P)H: quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating Sirt1 in diabetic nephropathy
Journal of Translational Medicine (2022)
-
CYP2E1-dependent upregulation of SIRT7 is response to alcohol mediated metastasis in hepatocellular carcinoma
Cancer Gene Therapy (2022)
-
The sirtuin family in health and disease
Signal Transduction and Targeted Therapy (2022)