Abstract
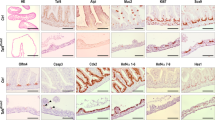
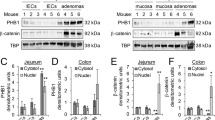
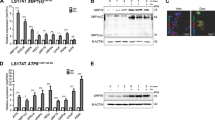
The enhancer of zeste homolog-2 (EZH2) represses gene transcription through histone H3 lysine-27-trimethylation (H3K27me3). Citrobacter rodentium (CR) promotes crypt hyperplasia and tumorigenesis by aberrantly regulating Wnt/β-catenin signaling. We aimed at investigating EZH2’s role in epigenetically regulating Wnt/β-catenin signaling following bacterial infection. NIH:Swiss outbred and ApcMin/+ mice were infected with CR (108 CFU); BLT1−/−ApcMin/+ mice, azoxymethane (AOM)/dextran sodium sulfate (DSS)-treated mice and de-identified human adenocarcinoma samples were the models of colon cancer. Following infection with wild-type but not mutant CR, elevated EZH2 levels in the crypt at days 6 and 12 (peak hyperplasia) coincided with increases in H3K27me3 and β-catenin levels, respectively. Chromatin immunoprecipitation revealed EZH2 and H3K27me3’s occupancy on WIF1 (Wnt inhibitory factor 1) promoter resulting in reduced WIF1 mRNA and protein expression. Following EZH2 knockdown via small interfering RNA or EZH2-inhibitor deazaneplanocin A (Dznep) either alone or in combination with histone deacetylase inhibitor suberoylanilide hydroxamic acid, WIF1 promoter activity increased significantly while the overexpression of EZH2 attenuated WIF1 reporter activity. Ectopic overexpression of SET domain mutant (F681Y) almost completely rescued WIF1 reporter activity and partially rescued WIF1 protein levels, whereas H3K27me3 levels were significantly attenuated suggesting that an intact methyltransferases activity is required for EZH2-dependent effects. Interestingly, although β-catenin levels were lower in EZH2-knocked down cells, F681Y mutants exhibited only partial reduction in β-catenin levels. Besides EZH2, increases in miR-203 expression in the crypts at days 6 and 12 post infection correlated with reduced levels of its target WIF1; overexpression of miR-203 in primary colonocytes decreased WIF1 mRNA and protein levels. Elevated levels of EZH2 and β-catenin with concomitant decrease in WIF1 expression in the polyps of CR-infected ApcMin/+ mice paralleled changes recorded in BLT1−/−ApcMin/+, AOM/DSS and human adenocarcinomas. Thus, EZH2-induced downregulation of WIF1 expression may partially regulate Wnt/β-catenin-dependent crypt hyperplasia in response to CR infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sasaki M, Ikeda H, Itatsu K, Yamaguchi J, Sawada S, Minato H et al. The overexpression of polycomb group proteins Bmi1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma. Lab Invest 2008; 88: 873–882.
Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet 2008; 40: 741–750.
Miranda TB, Cortez CC, Yoo CB, Liang G, Abe M, Kelly TK et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther 2009; 8: 1579–1588.
Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell 2011; 20: 187–199.
Taniguchi H, Jacinto FV, Villanueva A, Fernandez AF, Yamamoto H, Carmona FJ et al. Silencing of Kruppel-like factor 2 by the histone methyltransferase EZH2 in human cancer. Oncogene 2012; 31: 1988–1994.
Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008; 27: 7274–7284.
Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell 2012; 22: 506–523.
Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res 2011; 71: 225–233.
Clevers H, Nusse R . Wnt/beta-catenin signaling and disease. Cell 2012; 149: 1192–1205.
Chan DW, Chan CY, Yam JW, Ching YP, Ng IO . Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology 2006; 131: 1218–1227.
Sellin JH, Umar S, Xiao J, Morris AP . Increased beta-catenin expression and nuclear translocation accompany cellular hyperproliferation in vivo. Cancer Res 2001; 61: 2899–2906.
Umar S, Morris AP, Kourouma F, Sellin JH . Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif 2003; 36: 361–375.
Umar S, Wang Y, Sellin JH . Epithelial proliferation induces novel changes in APC expression. Oncogene 2005; 24: 6709–6718.
Umar S, Wang Y, Morris AP, Sellin JH . Dual alterations in casein kinase I-epsilon and GSK-3beta modulate beta-catenin stability in hyperproliferating colonic epithelia. Am J Physiol Gastrointest Liver Physiol 2007; 292: G599–G607.
Ahmed I, Chandrakesan P, Tawfik O, Xia L, Anant S, Umar S . Critical roles of Notch and Wnt/beta-catenin pathways in the regulation of hyperplasia and/or colitis in response to bacterial infection. Infect Immun 2012; 80: 3107–3121.
Chandrakesan P, Roy B, Jakkula LU, Ahmed I, Ramamoorthy P, Tawfik O et al. Utility of a bacterial infection model to study epithelial-mesenchymal transition, mesenchymal-epithelial transition or tumorigenesis. Oncogene 2013; 33: 2639–2654.
Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A et al. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci USA 2004; 101: 3597–3602.
Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 2003; 100: 11606–11611.
Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Cin Oncol 2006; 24: 268–273.
Sauvageau M, Sauvageau G . Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem cell 2010; 7: 299–313.
Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 2009; 11: 1487–1495.
Chandrakesan P, Ahmed I, Anwar T, Wang Y, Sarkar S, Singh P et al. Novel changes in NF-{kappa}B activity during progression and regression phases of hyperplasia: role of MEK, ERK, and p38. J Biol Chem 2010; 285: 33485–33498.
Jung HY, Jun S, Lee M, Kim HC, Wang X, Ji H et al. PAF and EZH2 induce Wnt/beta-catenin signaling hyperactivation. Mol Cell 2013; 52: 193–205.
Joshi P, Carrington EA, Wang L, Ketel CS, Miller EL, Jones RS et al. Dominant alleles identify SET domain residues required for histone methyltransferase of Polycomb repressive complex 2. J Biol Chem 2008; 283: 27757–27766.
Boffa LC, Vidali G, Mann RS, Allfrey VG . Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem 1978; 253: 3364–3366.
Ahmed I, Roy B, Chandrakesan P, Venugopal A, Xia L, Jensen R et al. Evidence of functional cross talk between the Notch and NF-kappaB pathways in nonneoplastic hyperproliferating colonic epithelium. Am J Physiol Gastrointest Liver Physiol 2013; 304: G356–G370.
Chandrakesan P, Jakkula LU, Ahmed I, Roy B, Anant S, Umar S . Differential effects of beta-catenin and NF-kappaB interplay in the regulation of cell proliferation, inflammation and tumorigenesis in response to bacterial infection. PLoS ONE 2013; 8: e79432.
Newman JV, Kosaka T, Sheppard BJ, Fox JG, Schauer DB . Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J Infect Dis 2001; 184: 227–230.
Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A . Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci 2007; 103: 24–32.
Hamon MA, Cossart P . Histone modifications and chromatin remodeling during bacterial infections. Cell Host Microbe 2008; 4: 100–109.
Sellin JH, Wang Y, Singh P, Umar S . beta-Catenin stabilization imparts crypt progenitor phenotype to hyperproliferating colonic epithelia. Exp Cell Res 2009; 315: 97–109.
Li X, Gonzalez ME, Toy K, Filzen T, Merajver SD, Kleer CG . Targeted overexpression of EZH2 in the mammary gland disrupts ductal morphogenesis and causes epithelial hyperplasia. Am J Pathol 2009; 175: 1246–1254.
Hayashi Y, Tsujii M, Wang J, Kondo J, Akasaka T, Jin Y et al. CagA mediates epigenetic regulation to attenuate let-7 expression in Helicobacter pylori-related carcinogenesis. Gut 2013; 62: 1536–1546.
Umar S . Citrobacter Infection and Wnt signaling. Curr Colorectal Cancer Rep 2012; 8: 298–306.
Shen L, Cui J, Liang S, Pang Y, Liu P . Update of research on the role of EZH2 in cancer progression. Onco Targets Ther 2013; 6: 321–324.
Cheng AS, Lau SS, Chen Y, Kondo Y, Li MS, Feng H et al. EZH2-mediated concordant repression of Wnt antagonists promotes beta-catenin-dependent hepatocarcinogenesis. Cancer Res 2011; 71: 4028–4039.
Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Patholl 2003; 201: 204–212.
Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer 2006; 94: 661–671.
Acknowledgements
We thank Dr Philip R Hardwidge (Department of Diagnostic Medicine/Pathobiology, College of Veterinary Medicine, Kansas State University, Manhattan, KS, USA) for Citrobacter rodentium mutants. This work was partially supported by the National Institutes of Health Grant R01 CA131413 and start-up funds from the University of Kansas Medical Center, Kansas City, KS, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Roy, B., Subramaniam, D., Ahmed, I. et al. Role of bacterial infection in the epigenetic regulation of Wnt antagonist WIF1 by PRC2 protein EZH2. Oncogene 34, 4519–4530 (2015). https://doi.org/10.1038/onc.2014.386
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.386
This article is cited by
-
Pertussis toxin-induced inhibition of Wnt/β-catenin signaling in dendritic cells promotes an autoimmune response in experimental autoimmune uveitis
Journal of Neuroinflammation (2023)
-
DCLK1 isoforms and aberrant Notch signaling in the regulation of human and murine colitis
Cell Death Discovery (2021)
-
Citrobacter rodentium alters the mouse colonic miRNome
Genes & Immunity (2019)
-
Barrett's esophagus is associated with a distinct oral microbiome
Clinical and Translational Gastroenterology (2018)
-
Structural Change in Microbiota by a Probiotic Cocktail Enhances the Gut Barrier and Reduces Cancer via TLR2 Signaling in a Rat Model of Colon Cancer
Digestive Diseases and Sciences (2016)