Abstract
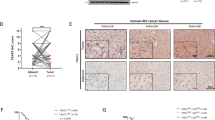
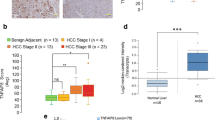
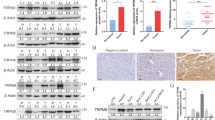
TIPE1 (tumor necrosis factor-α-induced protein 8-like 1 or TNFAIP8L1) is a newly identified member of the TIPE (TNFAIP8) family, which play roles in regulating cell death. However, the biologic functions of TIPE1 in physiologic and pathologic conditions are largely unknown. Here, we report the roles of TIPE1 in hepatocellular carcinoma (HCC). Evaluated by immunohistochemical staining, HCC tissues showed significantly downregulated TIPE1 expression compared with adjacent non-tumor tissues, which positively correlated with tumor pathologic grades and patient survival. Using a homograft tumor model in Balb/c mice, we discovered that TIPE1 significantly diminished the growth and tumor weight of murine liver cancer homografts. Consistently, TIPE1 inhibited both cell growth and colony formation ability of cultured HCC cell lines, which was further identified to be due to TIPE1-inducing apoptosis in a caspase-independent, necrostatin-1 (Nec-1)-insensitive manner. Furthermore, mechanistic investigations revealed that TIPE1 interacted with Rac1, and inhibited the activation of Rac1 and its downstream p65 and c-Jun N-terminal kinase pathway. Moreover, overexpression of constitutively active Rac1 partially rescued the apoptosis induced by TIPE1, and Rac1 knockdown significantly restored the deregulated cell growth induced by TIPE1 small interfering RNA. Our findings revealed that TIPE1 induced apoptosis in HCC cells by negatively regulating Rac1 pathway, and loss of TIPE1 might be a new prognostic indicator for HCC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 2012; 45: 487–498.
El-Serag HB, Rudolph KL . Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–2576.
Shiraha H, Yamamoto K, Namba M . Human hepatocyte carcinogenesis [review]. Int J Oncol 2013; 42: 1133–1138.
Fesik SW . Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 2005; 5: 876–885.
Delavallee L, Cabon L, Galan-Malo P, Lorenzo HK, Susin SA . AIF-mediated caspase-independent necroptosis: a new chance for targeted therapeutics. IUBMB Life 2011; 63: 221–232.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390.
Kumar D, Gokhale P, Broustas C, Chakravarty D, Ahmad I, Kasid U . Expression of SCC-S2, an antiapoptotic molecule, correlates with enhanced proliferation and tumorigenicity of MDA-MB 435 cells. Oncogene 2004; 23: 612–616.
Zhang C, Chakravarty D, Sakabe I, Mewani RR, Boudreau HE, Kumar D et al. Role of SCC-S2 in experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9 expression. Mol Ther 2006; 13: 947–955.
Dong QZ, Zhao Y, Liu Y, Wang Y, Zhang PX, Jiang GY et al. Overexpression of SCC-S2 correlates with lymph node metastasis and poor prognosis in patients with non-small-cell lung cancer. Cancer Sci 2010; 101: 1562–1569.
Miao Z, Zhao T, Wang Z, Xu Y, Song Y, Wu J et al. SCC-S2 is overexpressed in colon cancers and regulates cell proliferation. Tumour Biol 2012; 33: 2099–2106.
Shi TY, Cheng X, Yu KD, Sun M, Shao ZM, Wang M et al. Functional variants in TNFAIP8 associated with cervical cancer susceptibility and clinical outcomes. Carcinogenesis 2013; 34: 770–778.
Woodward MJ, de Boer J, Heidorn S, Hubank M, Kioussis D, Williams O et al. Tnfaip8 is an essential gene for the regulation of glucocorticoid-mediated apoptosis of thymocytes. Cell Death Differ 2010; 17: 316–323.
Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 2008; 133: 415–426.
Gus-Brautbar Y, Johnson D, Zhang L, Sun H, Wang P, Zhang S et al. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell 2012; 45: 610–618.
Sun H, Zhuang G, Chai L, Wang Z, Johnson D, Ma Y et al. TIPE2 controls innate immunity to RNA by targeting the phosphatidylinositol 3-kinase-Rac pathway. J Immunol 2012; 189: 2768–2773.
Wang Z, Fayngerts S, Wang P, Sun H, Johnson DS, Ruan Q et al. TIPE2 protein serves as a negative regulator of phagocytosis and oxidative burst during infection. Proc Natl Acad Sci USA 2012; 109: 15413–15418.
Gomez J, Martinez AC, Gonzalez A, Rebollo A . Dual role of Ras and Rho proteins: at the cutting edge of life and death. Immunol Cell Biol 1998; 76: 125–134.
Cui J, Zhang G, Hao C, Wang Y, Lou Y, Zhang W et al. The expression of TIPE1 in murine tissues and human cell lines. Mol Immunol 2011; 48: 1548–1555.
Wilson KD, Li Z, Wagner R, Yue P, Tsao P, Nestorova G et al. Transcriptome alteration in the diabetic heart by rosiglitazone: implications for cardiovascular mortality. PLoS One 2008; 3: e2609.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008; 135: 1311–1323.
Ma CH, Zhang Y, Wang XY, Gao LF, Liu H, Guo C et al. Human endostatin gene transfer, either naked or with liposome, has the same inhibitory effect on growth of mouse liver tumor cells in vivo. World J Gastroenterol 2004; 10: 2874–2877.
Yue X, Zhang Z, Liang X, Gao L, Zhang X, Zhao D et al. Zinc fingers and homeoboxes 2 inhibits hepatocellular carcinoma cell proliferation and represses expression of Cyclins A and E. Gastroenterology 2012; 142: 1559–1570.
Mathews MB, Bernstein RM, Franza BR Jr, Garrels JI . Identity of the proliferating cell nuclear antigen and cyclin. Nature 1984; 309: 374–376.
Sasi N, Hwang M, Jaboin J, Csiki I, Lu B . Regulated cell death pathways: new twists in modulation of BCL2 family function. Mol Cancer Ther 2009; 8: 1421–1429.
Brown JH, Del Re DP, Sussman MA The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res 2006; 98: 730–742.
Ozen C, Yildiz G, Dagcan A, Cevik D, Ors A, Keles U et al. Genetics and epigenetics of liver cancer. Nat Biotechnol 2013; 30: 381–384.
Filmus J, Capurro M . Glypican-3 and alphafetoprotein as diagnostic tests for hepatocellular carcinoma. Mol Diagn 2004; 8: 207–212.
Wennerberg K, Der CJ . Rho-family GTPases: it's not only Rac and Rho (and I like it). J Cell Sci 2004; 117: 1301–1312.
Etienne-Manneville S, Hall A . Rho GTPases in cell biology. Nature 2002; 420: 629–635.
Rossman KL, Der CJ, Sondek J . GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6: 167–180.
Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A . The diverse roles of Rac signaling in tumorigenesis. Cell Cycle 2011; 10: 1571–1581.
Joneson T, Bar-Sagi D . Suppression of Ras-induced apoptosis by the Rac GTPase. Mol Cell Biol 1999; 19: 5892–5901.
Chaigne-Delalande B, Anies G, Kramer I, Genot E . Nonadherent cells switch to a Rac-mediated, SHIP regulated, Akt activation mode for survival. Oncogene 2008; 27: 1876–1885.
Debidda M, Williams DA, Zheng Y . Rac1 GTPase regulates cell genomic stability and senescence. J Biol Chem 2006; 281: 38519–38528.
Edmondson HA, Steiner PE . Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954; 7: 462–503.
Ishak KG . Pathologic features of chronic hepatitis. A review and update. Am J Clin Pathol 2000; 113: 40–55.
Luan F, Liu H, Gao L, Liu J, Sun Z, Ju Y et al. Hepatitis B virus protein preS2 potentially promotes HCC development via its transcriptional activation of hTERT. Gut 2009; 58: 1528–1537.
Acknowledgements
This work was supported by grants from the National Science Foundation of China (Nos. 81172353, 81372211), Cultivating Project, Major Research Plan of National Natural Science Foundation of China (91129704), Programme for NCET-10-0524, Research Fund for the Doctoral Program of Higher Education of China (RFDP) (20110131110034) and Shandong Provincial Nature Science Foundation for Distinguished Young Scholars JQ200907.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Zhang, Z., Liang, X., Gao, L. et al. TIPE1 induces apoptosis by negatively regulating Rac1 activation in hepatocellular carcinoma cells. Oncogene 34, 2566–2574 (2015). https://doi.org/10.1038/onc.2014.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.208
This article is cited by
-
Transcriptome analysis of human cholangiocytes exposed to carcinogenic 1,2-dichloropropane in the presence of macrophages in vitro
Scientific Reports (2022)
-
TIPE1 inhibits osteosarcoma tumorigenesis and progression by regulating PRMT1 mediated STAT3 arginine methylation
Cell Death & Disease (2022)
-
TIPE1 inhibits the growth of Ewing’s sarcoma cells by suppressing Wnt/β-catenin signaling
Clinical and Translational Oncology (2022)
-
Decoding non-canonical mRNA decay by the endoplasmic-reticulum stress sensor IRE1α
Nature Communications (2021)
-
Human tumor necrosis factor alpha-induced protein eight-like 1 exhibited potent anti-tumor effect through modulation of proliferation, survival, migration and invasion of lung cancer cells
Molecular and Cellular Biochemistry (2021)