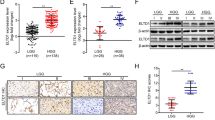
Abstract
Glioblastoma is the most common and aggressive primary brain tumor in adults, with a poor prognosis because of its resistance to radiotherapy and chemotherapy. Merlin/NF2 (moesin-ezrin-radixin-like protein/neurofibromatosis type 2) is a tumor suppressor found to be mutated in most nervous system tumors; however, it is not mutated in glioblastomas. Merlin associates with several transmembrane receptors and intracellular proteins serving as an anchoring molecule. Additionally, it acts as a key component of cell motility. By selecting sub-populations of U251 glioblastoma cells, we observed that high expression of phosphorylated Merlin at serine 518 (S518-Merlin), NOTCH1 and epidermal growth factor receptor (EGFR) correlated with increased cell proliferation and tumorigenesis. These cells were defective in cell-contact inhibition with changes in Merlin phosphorylation directly affecting NOTCH1 and EGFR expression, as well as downstream targets HES1 (hairy and enhancer of split-1) and CCND1 (cyclin D1). Of note, we identified a function for S518-Merlin, which is distinct from what has been reported when the expression of Merlin is diminished in relation to EGFR and NOTCH1 expression, providing first-time evidence that demonstrates that the phosphorylation of S518-Merlin in glioblastoma promotes oncogenic properties that are not only the result of inactivation of the tumor suppressor role of Merlin but also an independent process implicating a Merlin-driven regulation of NOTCH1 and EGFR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Fassl A, Tagscherer KE, Richter J, Berriel Diaz M, Alcantara Llaguno SR, Campos B et al. Notch1 signaling promotes survival of glioblastoma cells via EGFR-mediated induction of anti-apoptotic Mcl-1. Oncogene 2002; 31: 4698–4708.
Takebe N, Nguyen D, Yang SX . Targeting Notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol Ther. 2013; 141: 140–149.
Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 1993; 363: 515–521.
Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 1993; 72: 791–800.
Eldridge R . Central neurofibromatosis with bilateral acoustic neuroma. Adv Neurol 1981; 9: 57–65.
Bianchi AB, Hara T, Ramesh V, Gao J, Klein-Szanto AJ, Morin F et al. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet 1994; 6: 185–192.
Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q . Merlin is a potent inhibitor of glioma growth. Cancer Res 2008; 68: 5733–5742.
Houshmandi SS, Emnett RJ, Giovannini M, Gutmann DH . The neurofibromatosis 2 protein, merlin, regulates glial cell growth in an ErbB2- and Src-dependent manner. Mol Cel Biol 2009; 29: 1472–1486.
Stamenkovic I, Yu Q . Merlin, a ‘magic’ linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci 2010; 11: 471–484.
Pecina-Slaus N . Merlin, the NF2 gene product. Pathol Oncol Res 2013; 19: 365–373.
Sherman LS, Gutmann DH . Merlin: hanging tumor suppression on the Rac. Trends Cell Biol 2001; 11: 442–444.
Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 2010; 140: 477–490.
Surace EI, Haipek CA, Gutmann DH . Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene 2004; 23: 580–587.
Fernandez-Valle C, Tang Y, Ricard J, Rodenas-Ruano A, Taylor A, Hackler E et al. Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet 2002; 31: 354–362.
Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O . Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem 2004; 279: 18559–18566.
Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY et al. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol 2007; 9: 1199–1207.
Laulajainen M, Muranen T, Carpen O, Gronholm M . Protein kinase A-mediated phosphorylation of the NF2 tumor suppressor protein merlin at serine 10 affects the actin cytoskeleton. Oncogene 2008; 27: 3233–3243.
Gutmann DH, Haipek CA, Hoang Lu K . Neurofibromatosis 2 tumor suppressor protein, merlin, forms two functionally important intramolecular associations. J Neurosci Res 1999; 58: 706–716.
Sherman L, Xu HM, Geist RT, Saporito-Irwin S, Howells N, Ponta H et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene 1997; 15: 2505–2509.
Okada T, You L, Giancotti FG . Shedding light on Merlin's wizardry. Trends Cell Biol 2007; 17: 222–229.
Gronholm M, Muranen T, Toby GG, Utermark T, Hanemann CO, Golemis EA et al. A functional association between merlin and HEI10, a cell cycle regulator. Oncogene 2006; 25: 4389–4398.
Thaxton C, Lopera J, Bott M, Baldwin ME, Kalidas P, Fernandez-Valle C . Phosphorylation of the NF2 tumor suppressor in Schwann cells is mediated by Cdc42-Pak and requires paxillin binding. Mol Cell Neurosci 2007; 34: 231–242.
Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev 2001; 15: 968–980.
Okada M, Wang Y, Jang SW, Tang X, Neri LM, Ye K . Akt phosphorylation of merlin enhances its binding to phosphatidylinositols and inhibits the tumor-suppressive activities of merlin. Cancer Res 2009; 69: 4043–4051.
Kang CS, Zhang ZY, Jia ZF, Wang GX, Qiu MZ, Zhou HX et al. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther 2006; 13: 530–538.
Muranen T, Gronholm M, Renkema GH, Carpen O . Cell cycle-dependent nucleocytoplasmic shuttling of the neurofibromatosis 2 tumour suppressor merlin. Oncogene 2005; 24: 1150–1158.
Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI . Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol 2007; 177: 893–903.
Noda N, Honma S, Ohmiya Y . Hes1 is required for contact inhibition of cell proliferation in 3T3-L1 preadipocytes. Genes Cells 2011; 16: 704–713.
Mazzone M, Selfors LM, Albeck J, Overholtzer M, Sale S, Carroll DL et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci USA 2010; 107: 5012–5017.
Gonzales AJ, Fry DW . G1 cell cycle arrest due to the inhibition of erbB family receptor tyrosine kinases does not require the retinoblastoma protein. Exp Cell Res 2005; 303: 56–67.
Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W et al. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature 2011; 480: 118–122.
Jacoby LB, MacCollin M, Barone R, Ramesh V, Gusella JF . Frequency and distribution of NF2 mutations in schwannomas. Genes Chromosomes Cancer 1996; 17: 45–55.
Morrow KA, Das S, Metge BJ, Ye K, Mulekar MS, Tucker JA et al. Loss of tumor suppressor Merlin in advanced breast cancer is due to post-translational regulation. J Biol Chem 2011; 286: 40376–40385.
Lino MM, Merlo A, Boulay JL . Notch signaling in glioblastoma: a developmental drug target? BMC Med 2010; 8: 72.
Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol 2005; 25: 2384–2394.
Schulze KM, Hanemann CO, Muller HW, Hanenberg H . Transduction of wild-type merlin into human schwannoma cells decreases schwannoma cell growth and induces apoptosis. Hum Mol Genet 2002; 11: 69–76.
Maitra S, Kulikauskas RM, Gavilan H, Fehon RG . The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol 2006; 16: 702–709.
Visser-Grieve S, Hao Y, Yang X . Human homolog of Drosophila expanded, hEx, functions as a putative tumor suppressor in human cancer cell lines independently of the Hippo pathway. Oncogene 2011; 31: 1189–1195.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Acknowledgements
We are grateful to Drs F Giancotti and D Trono for providing vectors and reagents. We thank Jason Ngo, Andres Espinoza and Flavio Palalon for technical assistance. We also thank BCM Integrated Microscopy Core Facility at BCM supported by Shared Resources CPRIJ grant. The Characterized Cell Line Core at MDACC is supported by NCI Grant CA016672. This work was supported by NIH RO1 CA160335 (to DM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Guerrero, P., Yin, W., Camacho, L. et al. Oncogenic role of Merlin/NF2 in glioblastoma. Oncogene 34, 2621–2630 (2015). https://doi.org/10.1038/onc.2014.185
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.185
This article is cited by
-
MiR-1297 negatively regulates metabolic reprogramming in glioblastoma via repressing KPNA2
Human Cell (2020)
-
MicroRNA-335-5p is a potential suppressor of metastasis and invasion in gastric cancer
Clinical Epigenetics (2017)
-
Regulation of human glioma cell apoptosis and invasion by miR-152-3p through targeting DNMT1 and regulating NF2
Journal of Experimental & Clinical Cancer Research (2017)
-
Role of Merlin/NF2 inactivation in tumor biology
Oncogene (2016)