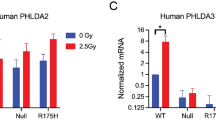
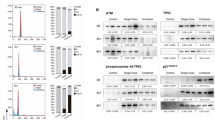
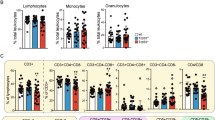
Abstract
Repeated low-dose γ-irradiation (IR) induces thymic lymphoma in mice because of oncogenic mutations propagating from a primitive hematopoietic stem/progenitor cell (HSC) in the bone marrow. It is well known that IR-induced thymic lymphomagenesis is markedly enhanced by p53 deficiency, yet data also indicate that p53-dependent apoptosis can actively drive tumor formation in this model. The latter was recently expounded on by findings from Puma-deficient mice, indicating that loss of this proapoptotic p53 target gene results in protection from IR-induced lymphomagenesis rather than enhanced susceptibility to. Similar to Puma, the transcription factor interferon regulatory factor 5 (Irf5) has been reported as a p53 target gene and is required for DNA damage-induced apoptosis. To date, no studies have been performed to elucidate the in vivo role of IRF5 in tumorigenesis. Given its essential role in DNA damage-induced apoptosis, we explored the tumor suppressor function of IRF5 in IR-induced thymic lymphomagenesis. Somewhat surprisingly, we found that thymic lymphoma development was significantly suppressed in Irf5−/− mice as compared with wild-type littermates. Suppression was due, in part, to reduced thymocyte and HSC apoptosis, resulting in reduced compensatory proliferation, and reduced replication stress-associated DNA damage. The observed effects were independent of p53 or Puma as these proteins were upregulated in Irf5−/− mice in response to IR. This study demonstrates an important new role for IRF5 in maintaining HSC homeostasis after IR and supports the non-redundant functions of IRF5, p53 and PUMA in DNA damage-induced lymphomagenesis. We propose that IRF5 may be an attractive target for developing therapeutic agents to ameliorate radiation-induced bone marrow injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rossi DJ, Jamieson CH, Weissman IL . Stem cells and the pathways to aging and cancer. Cell 2008; 132: 681–696.
Elledge SJ . Cell cycle checkpoints: preventing an identity crisis. Science 1996; 274: 1664–1672.
Vousden KH, Lane DP . P53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283.
Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 2006; 203: 2939–2951.
Michalak EM, Villunger A, Adam JM, Strasser A . In several cell types the tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 2008; 15: 1019–1029.
Kemp CJ, Wheldon T, Balmain A . P53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 1994; 8: 66–69.
Kelly PN, White MJ, Goschnick MW, Fairfax KA, Tarlinton DM, Kinkel SA et al. Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ 2010; 17: 1655–1664.
Michalak EM, Vandenberg CJ, Delbridge ARD, Wu L, Scott CL, Adams JM et al. Apoptosis-promoted tumorigenesis: γ-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev 2010; 24: 1608–1613.
Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J et al. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev 2010; 24: 1602–1607.
Mori T, Anazawa Y, Iiizumi M, Fukuda S, Nakamura Y, Arakawa H . Identification of the interferon regulatory factor 5 gene (IRF-5) as a direct target for p53. Oncogene 2002; 21: 2914–2918.
Barnes BJ, Kellum MJ, Pinder KE, Frisancho JA, Pitha PM . Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res 2003; 63: 6424–6431.
Hu G, Mancl ME, Barnes BJ . Signaling through IFN regulatory factor-5 sensitizes p53-deficient tumors to DNA damage-induced apoptosis and cell death. Cancer Res 2005; 65: 7403–7412.
Yanai H, Chen HM, Inuzuka T, Kondo S, Mak TW, Takaoka A et al. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc Natl Acad Sci USA 2007; 104: 3402–3407.
Stankovic T, Hubank M, Cronin D, Stewart GS, Fletcher D, Bignell CR et al. Microarray analysis reveals that TP53- and ATM-mutant B-CLLs share a defect in activating proapoptotic responses after DNA damage but are distinguished by major differences in activating prosurvival responses. Blood 2004; 103: 291–300.
Yang L, Zhao T, Shi X, Nakhaei P, Wang Y, Sun Q et al. Functional analysis of a dominant negative mutation of interferon regulatory factor 5. PLoS One 2009; 4: e5500.
Barnes BJ, Moore PA, Pitha PM . Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem 2001; 276: 23382–23390.
Watkins AJ, Hamoudi RA, Zeng N, Yan Q, Huang Y, Liu H et al. An integrated genomic and expression analysis of 7q deletion in splenic marginal zone lymphoma. PLoS One 2012; 7: e44997.
Fresquet V, Robles EF, Parker A, Martinez-Useros J, Mena M, Malumbres R et al. High-throughput sequencing analysis of the chromosome 7q32 deletion reveals IRF5 as a potential tumor supressor in splenic marginal-zone lymphoma. Br J Haematol 2012; 158: 712–726.
Kaplan HS, Brown MB . A quantitative dose–response study of lymphoid-tumor development in irradiated C57 black mice. J Natl Cancer Inst 1952; 13: 185–208.
Kaplan HS . The role of irradiation on experimental leukemogenesis. Natl Cancer Inst Monogr 1964; 14: 207–220.
Yang L, Feng D, Bi X, Stone RC, Barnes BJ . Monocytes from Irf5−/−mice have an intrinsic defect in their response to pristane-induced lupus. J Immunol 2012; 189: 3741–3750.
Kim HJ, Alonzo ES, Dorothee G, Pollard JW, Snat-Angelo DB . Selective depletion of eosinophils or neutrophils in mice impacts the efficiency of apoptotic cell clearance in the thymus. PLoS One 2010; 5: e11439.
Kominami R, Niwa O . Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci 2006; 97: 575–581.
Bi X, Hameed M, Mirani N, Pimenta EM, Anari J, Barnes BJ . Loss of interferon regulatory factor 5 (IRF5) expression in human ductal carcinoma correlates with disease stage and contributes to metastasis. Breast Cancer Res 2011; 13: R111.
Xu D, Meyer F, Ehlers E, Balsnitz L, Zhang L . Interferon regulatory factor 4 (IRF-4) targets IRF-5 to regulate Epstein–Barr virus transformation. J Biol Chem 2011; 286: 18261–18267.
Hu G, Barnes BJ . IRF-5 is a mediator of the death receptor-induced apoptotic signaling pathway. J Biol Chem 2009; 284: 2767–2777.
Botcheva K, McCorkle SR, McCombie WR, Dunn JJ, Anderson CW . Distinct p53 genomic binding patterns in normal and cancer-derived cell lines. Cell Cycle 2011; 10: 4237–4249.
Nikulenkov F, Spinnler C, Li H, Tonnelli C, Shi Y, Turunen M et al. Insights into p53 transcriptional function via genome-wide chromatin occupancy and gene expression analysis. Cell Death Differ 2012; 19: 1992–2002.
Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 2005; 123: 641–653.
Yu H, Shen H, Yuan Y, XuFeng R, Hu X, Garrison SP et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose γ-irradiation. Blood 2010; 115: 3472–3480.
Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995; 31: 1319–1339.
Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D . Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006; 107: 358–366.
Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mitzutani T et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 2005; 434: 243–249.
Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM et al. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor signaling. J Biol Chem 2005; 280: 17005–17012.
Feng D, Sangster-Guity N, Stone R, Korczeniewska J, Mancl ME, Fitzgerald-Bocarsly P et al. Differential requirement of histone acetylase and deacetylase activities for IRF5-mediated proinflammatory cytokine expression. J Immunol 2010; 185: 6003–6012.
Feng D, Yang L, Bi X, Stone RC, Patel P, Barnes BJ . Irf5-deficient mice are protected from pristane-induced lupus via increased Th2 cytokines and altered IgG class switching. Europ J Immunol 2012; 42: 1477–1487.
Liu C, Gao F, Li B, Mitchel REJ, Liu X, Lin J et al. TLR4 knockout protects mice from radiation-induced thymic lymphoma by downregulation of IL6 and miR-21. Leukemia 2011; 25: 1516–1519.
Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 2008; 28: 5391–5402.
Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684–696.
Purtha WE, Swiecki M, Colonna M, Diamond MS, Bhattacharya D . Spontaneous mutation of the Dock2 gene in Irf5–/– mice complicates interpretation of type I interferon production and antibody responses. Proc Natl Acad Sci USA 2012; 109: E898–E904.
Acknowledgements
We gratefully acknowledge Dr David Lagunoff for pathology assistance and Dr Lisong Yang for technical assistance. This work was supported in part by funds from the New Jersey Medical School-University Hospital Cancer Center, the UMDNJ Foundation and the New Jersey Commission on Cancer Research (NJCCR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bi, X., Feng, D., Korczeniewska, J. et al. Deletion of Irf5 protects hematopoietic stem cells from DNA damage-induced apoptosis and suppresses γ-irradiation-induced thymic lymphomagenesis. Oncogene 33, 3288–3297 (2014). https://doi.org/10.1038/onc.2013.295
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.295
Keywords
This article is cited by
-
Role of microRNAs and their downstream target transcription factors in zebrafish thrombopoiesis
Scientific Reports (2023)
-
Emerging roles of p53 and other tumour-suppressor genes in immune regulation
Nature Reviews Immunology (2016)