Abstract
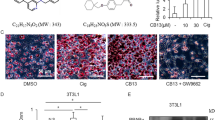
Peroxisome proliferator-activated receptor γ (PPARγ) may serve as a useful target for drug development in non-diabetic diseases. However, some colorectal cancer cells are resistant to PPARγ agonists by mechanisms that are poorly understood. Here, we provide the first evidence that elevated PPARδ expression and/or activation of PPARδ antagonize the ability of PPARγ to induce colorectal carcinoma cell death. More importantly, the opposing effects of PPARδ and PPARγ in regulating programmed cell death are mediated by survivin and caspase-3. We found that activation of PPARγ results in decreased survivin expression and increased caspase-3 activity, whereas activation of PPARδ counteracts these effects. Our findings suggest that PPARδ and PPARγ coordinately regulate cancer cell fate by controlling the balance between the cell death and survival and demonstrate that inhibition of PPARδ can reprogram PPARγ ligand-resistant cells to respond to PPARγ agonists.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Altieri DC . (2003). Validating survivin as a cancer therapeutic target. Nat Rev Cancer 3: 46–54.
Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D et al. (2000). Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res 91: 1204–1209.
Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP . (2003). Use of the peroxisome proliferator-activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Res Treat 79: 391–397.
Cellai I, Benvenuti S, Luciani P, Galli A, Ceni E, Simi L et al. (2006). Antineoplastic effects of rosiglitazone and PPARgamma transactivation in neuroblastoma cells. Br J Cancer 95: 879–888.
Di-Poi N, Michalik L, Tan NS, Desvergne B, Wahli W . (2003). The anti-apoptotic role of PPARbeta contributes to efficient skin wound healing. J Steroid Biochem Mol Biol 85: 257–265.
Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B . (2002). Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell 10: 721–733.
Farrow B, Evers BM . (2003). Activation of PPARgamma increases PTEN expression in pancreatic cancer cells. Biochem Biophys Res Commun 301: 50–53.
Fukuda S, Foster RG, Porter SB, Pelus LM . (2002). The antiapoptosis protein survivin is associated with cell cycle entry of normal cord blood CD34(+) cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood 100: 2463–2471.
Girnun GD, Smith WM, Drori S, Sarraf P, Mueller E, Eng C et al. (2002). APC-dependent suppression of colon carcinogenesis by PPARgamma. Proc Natl Acad Sci USA 99: 13771–13776.
Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN . (2001). Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J Biol Chem 276: 29681–29687.
Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN . (2004). Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med 10: 245–247.
Han S, Roman J . (2006). Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther 5: 430–437.
Hao CM, Redha R, Morrow J, Breyer MD . (2002). Peroxisome proliferator-activated receptor delta activation promotes cell survival following hypertonic stress. J Biol Chem 277: 21341–21345.
Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM . (2004). Peroxisome proliferator-activated receptor-delta attenuates colon carcinogenesis. Nat Med 10: 481–483.
He TC, Chan TA, Vogelstein B, Kinzler KW . (1999). PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99: 335–345.
Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M . (2002). Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem 277: 3247–3257.
Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC et al. (2006). PPARgamma insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-kappaB signaling pathway. Oncogene 25: 2736–2747.
Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N . (1998). Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 58: 5071–5074.
Kim EJ, Park KS, Chung SY, Sheen YY, Moon DC, Song YS et al. (2003). Peroxisome proliferator-activated receptor-gamma activator 15-deoxy-Delta12,14-prostaglandin J2 inhibits neuroblastoma cell growth through induction of apoptosis: association with extracellular signal-regulated kinase signal pathway. J Pharmacol Exp Ther 307: 505–517.
Kulke MH, Demetri GD, Sharpless NE, Ryan DP, Shivdasani R, Clark JS et al. (2002). A phase II study of troglitazone, an activator of the PPARgamma receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J 8: 395–399.
Kuo PC, Liu HF, Chao JI . (2004). Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J Biol Chem 279: 55875–55885.
Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K et al. (1998). Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med 4: 1053–1057.
Li M, Lee TW, Mok TS, Warner TD, Yim AP, Chen GG . (2005). Activation of peroxisome proliferator-activated receptor-gamma by troglitazone (TGZ) inhibits human lung cell growth. J Cell Biochem 96: 760–774.
Nadra K, Anghel SI, Joye E, Tan NS, Basu-Modak S, Trono D et al. (2006). Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol Cell Biol 26: 3266–3281.
O'Connor DS, Wall NR, Porter AC, Altieri DC . (2002). A p34(cdc2) survival checkpoint in cancer. Cancer Cell 2: 43–54.
Olie RA, Simoes-Wust AP, Baumann B, Leech SH, Fabbro D, Stahel RA et al. (2000). A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res 60: 2805–2809.
Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T et al. (2003). Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology 124: 361–367.
Panigrahy D, Huang S, Kieran MW, Kaipainen A . (2005). PPARgamma as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biol Ther 4: 687–693.
Park BH, Vogelstein B, Kinzler KW . (2001). Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA 98: 2598–2603.
Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C et al. (2000). Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol 20: 5119–5128.
Pino MV, Kelley MF, Jayyosi Z . (2004). Promotion of colon tumors in C57BL/6J-APC(min)/+ mice by thiazolidinedione PPARgamma agonists and a structurally unrelated PPARgamma agonist. Toxicol Pathol 32: 58–63.
Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM et al. (1998). Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med 4: 1058–1061.
Salvesen GS, Duckett CS . (2002). IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 3: 401–410.
Sarela AI, Scott N, Ramsdale J, Markham AF, Guillou PJ . (2001). Immunohistochemical detection of the anti-apoptosis protein, survivin, predicts survival after curative resection of stage II colorectal carcinomas. Ann Surg Oncol 8: 305–310.
Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB et al. (1998). Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med 4: 1046–1052.
Takayama O, Yamamoto H, Damdinsuren B, Sugita Y, Ngan CY, Xu X et al. (2006). Expression of PPARdelta in multistage carcinogenesis of the colorectum: implications of malignant cancer morphology. Br J Cancer 95: 889–895.
Teresi RE, Shaiu CW, Chen CS, Chatterjee VK, Waite KA, Eng C . (2006). Increased PTEN expression due to transcriptional activation of PPARgamma by Lovastatin and Rosiglitazone. Int J Cancer 118: 2390–2398.
Ueda Y, Wang S, Dumont N, Yi JY, Koh Y, Arteaga CL . (2004). Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility. J Biol Chem 279: 24505–24513.
Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN . (2005). Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res 65: 1822–1829.
Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q et al. (2006a). CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med 203: 941–951.
Wang D, Wang H, Guo Y, Ning W, Katkuri S, Wahli W et al. (2006b). Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proc Natl Acad Sci USA 103: 19069–19074.
Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B et al. (2004). Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell 6: 285–295.
Williams CW, Luongo C, Radhika A, Zhang T, Lamps LW, Nanney LB et al. (1996). Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology 111: 1134–1140.
Yin Y, Russell RG, Dettin LE, Bai R, Wei ZL, Kozikowski AP et al. (2005). Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res 65: 3950–3957.
Zaffaroni N, Daidone MG . (2002). Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions. Drug Resist Updat 5: 65–72.
Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ et al. (2001). Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res 61: 8664–8667.
Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR . (2000). The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci 113 (Part 23): 4363–4371.
Zuo X, Peng Z, Moussalli MJ, Morris JS, Broaddus RR, Fischer SM et al. (2009). Targeted genetic disruption of peroxisome proliferator-activated receptor-delta and colonic tumorigenesis. J Natl Cancer Inst 101: 762–767.
Acknowledgements
We thank the National Colorectal Cancer Research Alliance (NCCRA) for its generous support (RND). This work is supported, in part, by the NIH MERIT award R37 DK47297, RO1 DK 62112, NCI P01 CA77839 and CPRIT RP100960.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, D., Ning, W., Xie, D. et al. Peroxisome proliferator-activated receptor δ confers resistance to peroxisome proliferator-activated receptor γ-induced apoptosis in colorectal cancer cells. Oncogene 31, 1013–1023 (2012). https://doi.org/10.1038/onc.2011.299
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.299
Keywords
This article is cited by
-
Mechanisms of tRNA-derived fragments and tRNA halves in cancer treatment resistance
Biomarker Research (2020)
-
Unraveling the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) expression in colon carcinogenesis
npj Precision Oncology (2019)
-
Apoptotic effect of the selective PPARβ/δ agonist GW501516 in invasive bladder cancer cells
Tumor Biology (2016)
-
The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention
Nature Reviews Cancer (2012)