Abstract
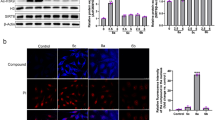
Sirtuin 1 (Sirt1) and Sirtuin 2 (Sirt2) belong to the family of NAD+ (nicotinamide adenine dinucleotide-positive)-dependent class III histone deacetylases and are involved in regulating lifespan. As cancer is a disease of ageing, targeting Sirtuins is emerging as a promising antitumour strategy. Here we present Salermide (N-{3-[(2-hydroxy-naphthalen-1-ylmethylene)-amino]-phenyl}-2-phenyl-propionamide), a reverse amide with a strong in vitro inhibitory effect on Sirt1 and Sirt2. Salermide was well tolerated by mice at concentrations up to 100 μM and prompted tumour-specific cell death in a wide range of human cancer cell lines. The antitumour activity of Salermide was primarily because of a massive induction of apoptosis. This was independent of global tubulin and K16H4 acetylation, which ruled out a putative Sirt2-mediated apoptotic pathway and suggested an in vivo mechanism of action through Sirt1. Consistently with this, RNA interference-mediated knockdown of Sirt1, but not Sirt2, induced apoptosis in cancer cells. Although p53 has been reported to be a target of Sirt1, genetic p53 knockdowns showed that the Sirt1-dependent proapoptotic effect of Salermide is p53-independent. We were finally able to ascribe the apoptotic effect of Salermide to the reactivation of proapoptotic genes epigenetically repressed exclusively in cancer cells by Sirt1. Taken together, our results underline Salermide's promise as an anticancer drug and provide evidence for the molecular mechanism through which Sirt1 is involved in human tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Avalos JL, Bever KM, Wolberger C . (2005). Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell 17: 855–868.
Bedalov A, Gatbonton T, Irvine WP, Gottschling DE, Simon JA . (2001). Identification of a small molecule inhibitor of Sir2p. Proc Natl Acad Sci USA 98: 15113–15118.
Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA . (2002). Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 277: 45099–45107.
Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB . (2005). Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 123: 437–448.
Clark AM, Labute P, Santavy M . (2006). 2D structure depiction. J Chem Inf Model 46: 1107–1123.
Finnin MS, Donigian JR, Pavletich NP . (2001). Structure of the histone deacetylase SIRT2. Nat Struct Biol 8: 621–625.
Ford J, Jiang M, Milner J . (2005). Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res 65: 10457–10463.
Fraga MF, Agrelo R, Esteller M . (2007). Cross-talk between aging and cancer: the epigenetic language. Ann N Y Acad Sci 1100: 60–74.
Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G et al. (2005). Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37: 391–400.
Fraga MF, Esteller M . (2007). Epigenetics and aging: the targets and the marks. Trends Genet 23: 413–418.
Glozak MA, Seto E . (2007). Histone deacetylases and cancer. Oncogene 26: 5420–5432.
Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL . (2001). Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem 276: 38837–38843.
Guarente L, Picard F . (2005). Calorie restriction—the SIR2 connection. Cell 120: 473–482.
Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S et al. (2006). Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res 66: 4368–4377.
Herranz M, Martin-Caballero J, Fraga MF, Ruiz-Cabello J, Flores JM, Desco M et al. (2006). The novel DNA methylation inhibitor zebularine is effective against the development of murine T-cell lymphoma. Blood 107: 1174–1177.
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al. (2003). Small molecule activators of Sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196.
Huhtiniemi T, Wittekindt C, Laitinen T, Leppanen J, Salminen A, Poso A et al. (2006). Comparative and pharmacophore model for deacetylase SIRT1. J Comput Aided Mol Des 20: 589–599.
Jones G, Willett P, Glen RC, Leach AR, Taylor R . (1997). Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267: 727–748.
Kamel C, Abrol M, Jardine K, He X, McBurney MW . (2006). SirT1 fails to affect p53-mediated biological functions. Aging Cell 5: 81–88.
Lain S, Hollick JJ, Campbell J, Staples OD, Higgins M, Aoubala M et al. (2008). Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell 13: 454–463.
Longo VD, Kennedy BK . (2006). Sirtuins in aging and age-related disease. Cell 126: 257–268.
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A et al. (2001). Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148.
Mai A, Massa S, Lavu S, Pezzi R, Simeoni S, Ragno R et al. (2005). Design, synthesis, and biological evaluation of sirtinol analogues as class III histone/protein deacetylase (Sirtuin) inhibitors. J Med Chem 48: 7789–7795.
Mariadason JM . (2008). HDACs and HDAC inhibitors in colon cancer. Epigenetics 3: 28–37.
Marks PA . (2007). Discovery and development of SAHA as an anticancer agent. Oncogene 26: 1351–1356.
Marks PA, Breslow R . (2007). Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 25: 84–90.
Min J, Landry J, Sternglanz R, Xu RM . (2001). Crystal structure of a SIR2 homolog–NAD complex. Cell 105: 269–279.
Minucci S, Pelicci PG . (2006). Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38–51.
Napper AD, Hixon J, McDonagh T, Keavey K, Pons JF, Barker J et al. (2005). Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem 48: 8045–8054.
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E . (2003). The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11: 437–444.
Olaharski AJ, Rine J, Marshall BL, Babiarz J, Zhang L, Verdin E et al. (2005). The flavoring agent dihydrocoumarin reverses epigenetic silencing and inhibits sirtuin deacetylases. PLoS Genet 1: e77.
Onufriev A, Bashford D, Case DA . (2004). Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 55: 383–394.
Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M et al. (2006). Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene 25: 176–185.
Parks BA, Jiang L, Thomas PM, Wenger CD, Roth MJ, Boyne II MT et al. (2007). Top-down proteomics on a chromatographic time scale using linear ion trap Fourier transform hybrid mass spectrometers. Anal Chem 79: 7984–7991.
Ponder JW, Case DA . (2003). Force fields for protein simulations. Adv Protein Chem 66: 27–85.
Pruitt K, Zinn RL, Ohm JE, McGarvey KM, Kang SH, Watkins DN et al. (2006). Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet 2: e40.
Rodrigues NR, Rowan A, Smith ME, Kerr IB, Bodmer WF, Gannon JV et al. (1990). p53 mutations in colorectal cancer. Proc Natl Acad Sci USA 87: 7555–7559.
Ropero S, Menendez JA, Vazquez-Martin A, Montero S, Cortes-Funes H, Colomer R . (2004). Trastuzumab plus tamoxifen: anti-proliferative and molecular interactions in breast carcinoma. Breast Cancer Res Treat 86: 125–137.
Stunkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Salto-Tellez M et al. (2007). Function of the SIRT1 protein deacetylase in cancer. Biotechnol J 2: 1360–1368.
Sun Y, Sun D, Li F, Tian L, Li C, Li L et al. (2007). Downregulation of Sirt1 by antisense oligonucleotides induces apoptosis and enhances radiation sensitization in A549 lung cancer cells. Lung Cancer 58: 21–29.
Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D . (2004). Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 16: 93–105.
Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW et al. (2006). SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 20: 1256–1261.
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK et al. (2001). hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159.
Villar-Garea A, Israel L, Imhof A . (2008). Analysis of histone modifications by mass spectrometry. Curr Protoc Prot Sci (Chapter 14, Unit 14 10).
Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y et al. (2006). Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol 8: 1025–1031.
Xu WS, Parmigiani RB, Marks PA . (2007). Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26: 5541–5552.
Acknowledgements
This work was primarily supported by the European Union (LSHG-CT-2006-037415). MFF is funded by the Spanish Ramon & Cajal Programme and the Health Department of the Spanish Government (PI061267). Thanks are due to PRIN2006 (AM) and AICR2007 (AM) for grants. The Cancer Epigenetics group at the CNIO is supported by the Health (FIS01-04) and Education and Science (I+D+I MCYT08-03, FU2004-02073/BMC and Consolider MEC09-05) Departments of the Spanish Government, the European Grant TRANSFOG LSHC-CT-2004-503438, EPITRON LSHC-CT-2005-518417, APO-SYS HEALTH-F4-2007-200767 and the Spanish Association Against Cancer (AECC). EL is a recipient of a fellowship from the FIS Spanish Research Program. VC is a recipient of a Fellowship from the FPU Spanish Research Program.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Rights and permissions
About this article
Cite this article
Lara, E., Mai, A., Calvanese, V. et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene 28, 781–791 (2009). https://doi.org/10.1038/onc.2008.436
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.436
Keywords
This article is cited by
-
Sirtuin inhibition is synthetic lethal with BRCA1 or BRCA2 deficiency
Communications Biology (2021)
-
Chronic Exercise Mitigates the Effects of Sirtuin Inhibition by Salermide on Endothelium-Dependent Vasodilation
Cardiovascular Toxicology (2021)
-
A novel chemical-combination screen in zebrafish identifies epigenetic small molecule candidates for the treatment of Duchenne muscular dystrophy
Skeletal Muscle (2020)
-
Lysine acetylation as drug target in fungi: an underexplored potential in Aspergillus spp.
Brazilian Journal of Microbiology (2020)
-
Inhibition of SIRT1/2 upregulates HSPA5 acetylation and induces pro-survival autophagy via ATF4-DDIT4-mTORC1 axis in human lung cancer cells
Apoptosis (2019)