Abstract
Effector triggered conformational changes of proteins such as regulators of transcription, receptors, or enzymes are the molecular basis for regulation in biology. Most proteins perform their biological functions intracellularly, in the presence of many potential interaction partners. Studies of conformational changes have mainly been performed in vitro using sophisticated physical and biochemical methods that usually require purified proteins. Here we describe the observation of conformational changes of Tet repressor in the cytoplasm of growing Escherichia coli cells, analyzed by ligand dependent disulfide crosslinking of cysteine residues substituted into mobile regions of the protein. The amount of protein undergoing the structural change is quantitatively linked to the concomitant induction of transcription of a reporter gene.
Similar content being viewed by others
Main
Regulation of gene expression depends on proteins that recognize signals and respond to their presence with altered activities. Tetracycline dependent gene regulation, which was identified in bacteria, is now widely used as a tool to control the expression of single genes in many different organisms1. The tetracycline repressor (TetR) is one of the most intensely studied repressors, and this system has served as a paradigm for regulators that undergo conformational changes between inducer bound and DNA bound forms. In the absence of the inducer tetracycline, TetR binds to the tet operators (tetO), repressing the resistance gene tetA. Upon binding inducer, however, the operator binding activity of TetR is abolished which leads to expression of the resistance, a H+-tetracycline antiporter2. Crystallographic3,4, spectroscopic5, and biochemical analyses6,7 have demonstrated that TetR undergoes a conformational change upon binding tetracycline that is believed to be essential for induction of transcription.
We have recently constructed mutants of TetR that contain pairs of cysteine residues which can form intermonomer disulfide bonds and thus form dimers under oxidizing conditions. These mutants can be used to characterize structural changes. Pairs of cysteine residues were placed at positions in which only one conformation of TetR would allow disulfide bond formation7, and in vitro oxidation results were in accordance with proposed movements in discrete regions of the protein8. Thus, the formation of specific disulfide bonds indicates the conformational state of these TetR mutants. If these disulfides could be formed in the cytoplasm of a bacterial cell, it would be possible to use disulfide crosslinking to detect structural changes of TetR in vivo and to correlate them to induction of transcription. However, disulfides are not readily formed in E. coli because the cytoplasm is a reducing environment.
Disulfide formation has been used previously as an indicator of the topology and/or structural changes in studies of membrane proteins such as chemoreceptors and lactose permease. In these experiments, an environment that could allow disulfide formation was obtained by making use of isolated membranes, thiol specific linkers, or whole E. coli cells that had been oxidized by treatment with iodine or copper phenanthroline9,10,11,12. However, we have taken a different approach here. The reducing environment in E. coli depends on the activities of the thioredoxin and glutathione/glutaredoxin pathways13. Inactivation of enzymes in one or both of these pathways have yielded strains that have a more oxidizing environment in the cytoplasm, as indicated by the activity of particular enzymes, such as alkaline phosphatase and urokinase, that require the formation of disulfides for their activities14,15,16. Here we utilize a strain with a disruption in the gor gene that lacks a functional glutathione reductase to enable the formation of disulfides in vivo.
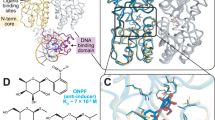
The TetR variants used are listed in Table 1, and the locations of the cysteines in the X-ray structure of the inducer bound form of TetR are shown in Fig. 1. It is essential for this analysis that the repressor activity of each TetR mutant be unchanged in E. coli, and so in Table 1 we also present the degree of induction of a tetA–lacZ fusion by each of the TetR cysteine mutants. Five of the six mutants are inducible to a similar degree, and these five were used in further analysis of disulfide bond formation.
The two monomers are shown as blue and green ribbons and tetracycline as a yellow stick model. The recognition helices α3 and α3′ of both helix-turn-helix motifs are red. Cysteine residues are designated and colored as follows: I22C/I22C′ (d1, yellow) and D23C/D23C′ (d2, red); P159C/D106C′ (d3, orange) and N165C/E107C′ (d4, magenta); L142C/D143C′ (d5, pink) and R195C/V199C′ (d6, orange).
An E. coli strain carrying a gor:spc disruption (strain IMW200, kindly provided by G. Unden, Mainz) was transformed with the mutated tetR genes and grown in the presence of 0.4 μM tetracycline to enable the formation of an inducer bound TetR complex. A complex of TetR with the operator was formed in the absence of tetracycline by cotransformation of the high copy number plasmid pWH707 carrying seven copies of tetO. The strains were grown to stationary phase, and soluble crude protein extracts were prepared and analyzed using non-reducing SDS-PAGE. Visualization was performed by western blotting with a mixture of monoclonal antibodies6 (kindly provided by E. Pook and S. Grimm, Erlangen). Since disulfide bond formation leads to a covalent linkage between the two monomers in these mutant TetR dimers, the crosslinked forms migrate as dimers and the non-crosslinked forms as monomers.
The extent of in vivo crosslinking in the TetR mutants is shown in Fig. 2. When they were expressed in an otherwise isogenic E. coli strain containing a functional gor gene, no dimers were detected (Fig. 2f). Thus, disulfides were apparently not formed during the preparation of the crude protein extracts, but occurred in the different redox environment in the cytoplasm of E. coli IMW200. Dimers are formed in a ligand dependent manner (where the ligand that induces the conformation could be tetracycline or DNA) by TetR D23C/D23C′ (Fig. 2b; the amino acid from the second monomer is indicated by a prime), D106C/P159C′ (Fig. 2c), and E107C/N165C′ (Fig. 2d), whereas TetR I22C/I22C′ (Fig. 2a) and R195C/V199C′ (Fig. 2e) contain roughly the same amounts of dimer regardless of the presence or absence of ligands. We conclude that the disulfide bonds in TetR I22C/I22C′ and R195C/V199C′ have the same chance of being formed in the free, tetracycline bound, and DNA bound conformations. TetR I22C/I22C′ appears to be primarily monomeric, and therefore it appears that this disulfide bond cannot be formed in vivo at all. The monomeric form of D23C/D23C′ containing cysteine residues in the DNA binding region, the so-called DNA reading heads, is present in lower amounts when bound to tetracycline compared when bound to the operator (Fig. 2b). This reflects the larger distance between the cysteine residues at this position when TetR is bound to the operator. This is in agreement with X-ray3,4 and EPR analyses5: the crystal structures of the DNA bound and tetracycline bound proteins show a 6.8 Å difference in distance between the Cα atoms of the residues at position 23 in each monomer3,4 (Fig. 3). The mutants TetR D106C/P159C′ (Fig. 2c) and E107C/N165C′ (Fig. 2d) show partial dimer formation in the operator bound but not the tetracycline bound form. This resembles the respective in vitro results7 and indicates a movement of the loop between α-helices 8 and 9. Thus, the conformational changes of the DNA reading head and the flexible loop, which reflect the closure of the tetracycline binding pocket after binding of the drug8,17, also seem to occur in vivo.
Cells of E. coli IMW200 (gor:spc) carrying the tetR mutants a, I22C, b, D23C, c, D106C/P159C, d, E107C/N165C, e, R195C/V199C. f, tetR E107C/N165C in an otherwise isogenic E. coli strain containing the functional gor. All were grown in the presence of 0.4 μM tetracycline, in the absence of tetracycline but cotransformed with a plasmid carrying seven copies of tet operator (O) or without a ligand (−). The migration of monomers (M, non-crosslinked) and dimers (D, crosslinked) are indicated. Since two disulfide bonds per dimer of TetR D106C/P159C′ and E107C/N165C′ can be formed the double bands may reflect dimers containing one or two crosslinks, respectively7.
a, An overlay is shown of the 60 N-terminal amino acids in the TetR–([Mg-tetracycline]+)2 (blue ribbon) and the TetR–tetO (green ribbon) structures. The recognition helices α3 and α3′ are light red (tetracycline) and dark red (DNA) and the N-termini are indicated. The cysteine residues at positions 22 and 23 are colored (I22C tetracycline and I22C′ tetracycline, orange; I22C DNA and I22C′ DNA, dark red; D23C tetracycline and D23C′ tetracycline, red; D23C DNA and D23C′ DNA, magenta). Only D23C is labeled. b, Distances between the Cα atoms of the cysteine residues.
The agreement of results showing the efficiency of disulfide bond formation in vitro and in vivo directly demonstrates for the first time that the conformational changes seen in the isolated protein in solution and in the crystal structure also occur in the environment of the undisrupted, growing E. coli cell. While this has been assumed for a long time, until now it has been very difficult to obtain experimental evidence to support this idea.
The ability to assess structural changes in vivo by disulfide bond formation prompted us to seek a quantitative correlation between the structural change that TetR undergoes upon binding inducer and induction of expression of a tet operator in vivo. For this purpose, a tetA-gfp indicator fusion gene18 was introduced into E. coli IMW200. This fusion allows green fluorescent protein (GFP) expression to be controlled by TetR E107C/N165C, which shows dimer formation when bound to tetO but not when bound to tetracycline. The multicopy plasmid with seven copies of tetO was also transformed into this strain and the amount of tetracycline in the broth was gradually increased. For each tetracycline concentration, GFP expression was quantified and samples were analyzed for the amount of dimer formed (Fig. 4). The gradual formation of the inducer bound structure of TetR parallels induction of GFP expression, although the formation of the inducer bound structure at low tetracycline concentrations is somewhat more efficient than induction. This may reflect the fact that TetR is present in excess over tetO, hence some TetR may shift to the inducer bound form at low tetracycline concentrations without having much effect on expression. When nearly complete tetO induction is reached, all of TetR appears to be in the inducer bound form. Thus, we demonstrate here that the conformational change of TetR parallels its functional change with respect to the in vivo induction of tet operator expression. This supports the assumption that the conformational change is a prerequisite for in vivo induction.
Panel (b) shows a western blot of non-reducing SDS PAGE of soluble protein extracts from E. coli IMW200 carrying tetR E107C/N165C, the plasmid with seven copies of tetO and a tetA–gfp fusion grown in the presence of increasing amounts of tetracycline as indicated. The locations of monomers (M, non-crosslinked) and dimers (D, crosslinked) are indicated. In panel (a), the fraction of non-crosslinked TetR, which is indicative of the inducer bound structure (see Fig. 2d) was quantified (filled circles) and is plotted as the percentage of total TetR versus tetracycline concentration in the upper part. The repression efficiency was determined from GFP fluorescence at each tetracycline concentration (triangles in (a)).
Methods
Plasmids.
The construction of the tetR mutants has been described5,7. For construction of pWH707 a 397 base pair BamHI-EcoRI fragment from pTop10 GUS19 carrying seven copies of the tet operator was ligated in the likewise digested vector pMc5-8 (ref. 20).
Disulfide bond formation.
Cells of E. coli IMW200 were inoculated from a preculture into 15 ml Luria Bertani broth supplemented with the appropiate antibiotics in a 100 ml flask and incubated on a shaker at 37 °C for 20 h. The cells were then placed for one hour on ice and centrifuged. The pellet was resuspended in 100 mM iodoacetamide, 1.5% (w/v) sodium dodecyl sulfate, 5 mM EDTA and 100 mM Tris-HCl pH 8.8 and the mixture sonicated (30 s, 30 W) and centrifuged. The supernatant was then analyzed in non-reducing SDS-PAGE7 and TetR was visualized by western blotting.
References
Forster, K. et al. Nucleic Acids Res. 27, 708–710 (1999).
Hillen, W. & Berens, C. Annu. Rev. Microbiol. 48, 345–369 (1994).
Hinrichs, W. et al. Science 264, 418–420 (1994).
Orth, P., Schnappinger, D., Hillen, W., Saenger, W. & Hinrichs, W. Nature Struct. Biol. 7, 215–219 (2000).
Tiebel, B. et al. J. Mol. Biol. 290, 229–240 (1999).
Pook, E., Grimm, S., Bonin, A., Winkler, T. & Hillen, W. Eur. J. Biochem. 258, 915–922 (1998).
Tiebel, B., Aung-Hilbrich, L.M., Schnappinger, D. & Hillen, W. EMBO J. 17, 5112–5119 (1998).
Müller, G. et al. Nature Struct. Biol. 2, 693–703 (1995).
Lee, G.F., Lebert, M.R., Lilly, A.A. & Hazelbauer, G.L. Proc. Natl. Acad. Sci. USA 92, 3391–3395 (1995).
Hughson, A.G., Lee, G.F. & Hazelbauer, G.L. Protein Sci. 6, 315–322 (1997).
Wu, J., Hardy, D. & Kaback, H.R. Biochemistry 37, 15785–15790 (1998).
Bass, R.B. & Falke, J.J. Structure Fold. Des. 7, 829–840 (1999)
Rietsch, A. & Beckwith, J. Annu. Rev. Genet. 32, 163–184 (1998).
Derman, A.I., Prinz, W.A., Belin, D. & Beckwith, J. Science 262, 1744–1747 (1993).
Prinz, W., Aslund, F., Holmgren, A. & Beckwith, J. J. Biol. Chem. 272, 15661–15667 (1997).
Bessette, P.H., Aslund, F., Beckwith, J. & Georgiou, G. Proc. Natl. Acad. Sci. USA 96, 13703–13708 (1999).
Orth, P. et al. J. Mol. Biol. 279, 439–447 (1998).
Scholz, O., Thiel, A., Hillen, W. & Niederweis, M. Eur. J. Biochem. 267, 1–7 (2000).
Weinmann, P. et al. Plant J. 4, 559–569 (1994).
Stanssens, P. et al. Nucleic Acids Res. 17, 4441–4454 (1989).
Miller, J.H. Experiments in molecular genetics (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York; 1972).
Acknowledgements
We thank G. Unden for the E. coli strain IMW200 and E. Pook and S. Grimm for monoclonal antibodies. This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie. B.T. was a recipient of a personal grant from the Boehringer Ingelheim Fonds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiebel, B., Garke, K. & Hillen, W. Observing conformational and activity changes of Tet repressor in vivo. Nat Struct Mol Biol 7, 479–481 (2000). https://doi.org/10.1038/nsb0600_479
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0600_479