Key Points
-
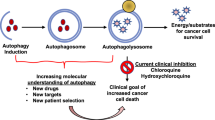
Autophagy is a dynamic metabolic process that facilitates nutrient utilization and toxin removal: too little or too much autophagy can have beneficial or deleterious effects depending on the context
-
Understanding the context-dependent role of autophagy in cancer development, and specifically in treatment resistance, has the potential to improve current treatment of advanced prostate cancer
-
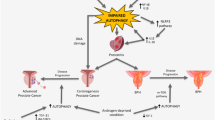
Preclinical prostate cancer models show significant upregulation of autophagy in response to androgen deprivation therapy, taxane-based chemotherapy, targeted inhibition of kinases, and amino acid restriction
-
Adjuvant autophagy inhibition in preclinical prostate cancer models improves cell killing and tumour responsiveness to treatment
-
Several autophagic modulators are under active investigation in clinical trials, including chloroquine and hydroxychloroquine
-
Currently available autophagy modulators are relatively nonspecific, and cytotoxicity in noncancerous tissues is a concern: moving forward, refinement of autophagy modulation is needed
Abstract
Autophagy, or 'self-eating', is an adaptive process that enables cells to cope with metabolic, toxic, and even infectious stressors. Although the adaptive capability of autophagy is generally considered beneficial, autophagy can also enhance nutrient utilization and improve growth characteristics of cancer cells. Moreover, autophagy can promote greater cellular robustness in the context of therapeutic intervention. In advanced prostate cancer, preclinical data provide evidence that autophagy facilitates both disease progression and therapeutic resistance. Notably, androgen deprivation therapy, taxane-based chemotherapy, targeted kinase inhibition, and nutrient restriction all induce significant cellular distress and, subsequently, autophagy. Understanding the context-dependent role of autophagy in cancer development and treatment resistance has the potential to improve current treatment of advanced prostate cancer. Indeed, preclinical studies have shown that the pharmacological inhibition of autophagy (with agents including chloroquine, hydroxychloroquine, metformin, and desmethylclomipramine) can enhance the cell-killing effect of cancer therapeutics, and a number of these agents are currently under investigation in clinical trials. However, many of these autophagy modulators are relatively nonspecific, and cytotoxicity in noncancerous tissues is still a concern. Moving forward, refinement of autophagy modulation is needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bennett, H. L. et al. Does androgen-ablation therapy (AAT) associated autophagy have a pro-survival effect in LNCaP human prostate cancer cells? BJU Int. 111, 672–682 (2013).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Xie, Z. & Klionsky, D. J. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 (2007).
Degenhardt, K. et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10, 51–64 (2006).
Leone, R. D. & Amaravadi, R. K. Autophagy: a targetable linchpin of cancer cell metabolism. Trends Endocrinol. Metab. 24, 209–217 (2013).
Chen, N. & Karantza, V. Autophagy as a therapeutic target in cancer. Cancer Biol. Ther. 11, 157–168 (2011).
Chodak, G. W. & Warren, K. S. Watchful waiting for prostate cancer: a review article. Prostate Cancer Prostatic Dis. 9, 25–29 (2006).
Patel, A. R. & Klein, E. A. Risk factors for prostate cancer. Nat. Clin. Pract. Urol. 6, 87–95 (2009).
Cheong, H., Lu, C., Lindsten, T. & Thompson, C. B. Therapeutic targets in cancer cell metabolism and autophagy. Nat. Biotechnol. 30, 671–678 (2012).
Deretic, V. Autophagosome and phagosome. Methods Mol. Biol. 445, 1–10 (2008).
Townsend, K. N. et al. Autophagy inhibition in cancer therapy: metabolic considerations for antitumor immunity. Immunol. Rev. 249, 176–194 (2012).
Deretic, V. Autophagy: an emerging immunological paradigm. J. Immunol. 189, 15–20 (2012).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Zhang, Y., Morgan, M. J., Chen, K., Choksi, S. & Liu, Z. G. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 119, 2895–2905 (2012).
Choi, A. M., Ryter, S. W. & Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 368, 1845–1846 (2013).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544 (2012).
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003).
Mathew, R., Karantza-Wadsworth, V. & White, E. Role of autophagy in cancer. Nat. Rev. Cancer 7, 961–967 (2007).
White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 12, 401–410 (2012).
Lum, J. J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005).
Yonekawa, T. & Thorburn, A. Autophagy and cell death. Essays Biochem. 55, 105–117 (2013).
Mariño, G., Niso-Santano, M., Baehrecke, E. H. & Kroemer, G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94 (2014).
Levine, B. & Yuan, J. Autophagy in cell death: an innocent convict? J. Clin. Invest. 115, 2679–2688 (2005).
Mehrpour, M., Esclatine, A., Beau, I. & Codogno, P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 20, 748–762 (2010).
Pattingre, S. et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122, 927–939 (2005).
Kondo, Y., Kanzawa, T., Sawaya, R. & Kondo, S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 5, 726–734 (2005).
Yang, Z. J., Chee, C. E., Huang, S. & Sinicrope, F. Autophagy modulation for cancer therapy. Cancer Biol. Ther. 11, 169–176 (2011).
Carew, J. S. et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 110, 313–322 (2007).
Katayama, M., Kawaguchi, T., Berger, M. S. & Pieper, R. O. DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ. 14, 548–558 (2007).
White, E. & DiPaola, R. S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 15, 5308–5316 (2009).
Amaravadi, R. K. et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 17, 654–666 (2011).
Yang, Z. J., Chee, C. E., Huang, S. & Sinicrope, F. A. The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 10, 1533–1541 (2011).
Sotelo, J., Briceño, E. & López-González, M. A. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 144, 337–343 (2006).
Vakana, E., Altman, J. K. & Platanias, L. C. Targeting AMPK in the treatment of malignancies. J. Cell. Biochem. 113, 404–409 (2012).
Ben Sahra, I. et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 70, 2465–2475 (2010).
Ben Sahra, I. et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 27, 3576–3586 (2008).
Cantrell, L. A. et al. Metformin is a potent inhibitor of endometrial cancer cell proliferation—implications for a novel treatment strategy. Gynecol. Oncol. 116, 92–98 (2010).
Cazzaniga, M., Bonanni, B., Guerrieri-Gonzaga, A. & Decensi, A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol. Biomarkers Prev. 18, 701–705 (2009).
Colquhoun, A. J. et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 15, 346–352 (2012).
Zakikhani, M., Dowling, R., Fantus, I. G., Sonenberg, N. & Pollak, M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 66, 10269–10273 (2006).
Shank, J. J. et al. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol. Oncol. 127, 390–397 (2012).
Zhang, Y. et al. Effects of metformin on CD133+ colorectal cancer cells in diabetic patients. PLoS ONE 8, e81264 (2013).
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R. & Morris, A. D. Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 (2005).
Spratt, D. E. et al. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur. Urol. 63, 709–716 (2013).
Rossi, M. et al. Desmethylclomipramine induces the accumulation of autophagy markers by blocking autophagic flux. J. Cell Sci. 122, 3330–3339 (2009).
Nguyen, H. G. et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene http://dx.doi.org/10.1038/onc.2014.25.
Kung, H.-J. et al. in Prostate Cancer, (ed. Tindall, D. J.) 497–518 (Springer, 2013).
Shen, S. et al. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene 30, 4544–4556 (2011).
Giampietri, C. et al. Autophagy modulators sensitize prostate epithelial cancer cell lines to TNF-alpha-dependent apoptosis. Apoptosis 17, 1210–1222 (2012).
Bennett, H. L., Fleming, J. T., O'Prey, J., Ryan, K. M. & Leung, H. Y. Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cells. Cell Death Dis. 1, e72 (2010).
Kaini, R. R. & Hu, C. A. Synergistic killing effect of chloroquine and androgen deprivation in LNCaP cells. Biochem. Biophys. Res. Commun. 425, 150–156 (2012).
Li, M. et al. Autophagy protects LNCaP cells under androgen deprivation conditions. Autophagy 4, 54–60 (2008).
Jiang, Q. et al. Targeting androgen receptor leads to suppression of prostate cancer via induction of autophagy. J. Urol. 188, 1361–1368 (2012).
Xu, Y., Chen, S. Y., Ross, K. N. & Balk, S. P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 66, 7783–7792 (2006).
Chhipa, R. R., Wu, Y. & Ip, C. AMPK-mediated autophagy is a survival mechanism in androgen-dependent prostate cancer cells subjected to androgen deprivation and hypoxia. Cell. Signal. 23, 1466–1472 (2011).
Lozy, F. & Karantza, V. Autophagy and cancer cell metabolism. Semin. Cell Dev. Biol. 23, 395–401 (2012).
Ziparo, E. et al. Autophagy in prostate cancer and androgen suppression therapy. Int. J. Mol. Sci. 14, 12090–12106 (2013).
Barrett, K. E., Boitano, S., Barman, S. M. & Brooks, H. L. Ganong's Review of Medical Physiology 23rd edn Ch. 2 (McGraw-Hill, 2012).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Köchl, R., Hu, X. W., Chan, E. Y. & Tooze, S. A. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 7, 129–145 (2006).
Viola, G. et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem. Pharmacol. 83, 16–26 (2012).
Veldhoen, R. A. et al. The chemotherapeutic agent paclitaxel inhibits autophagy through two distinct mechanisms that regulate apoptosis. Oncogene 32, 736–746 (2013).
Notte, A., Ninane, N., Arnould, T. & Michiels, C. Hypoxia counteracts taxol-induced apoptosis in MDA-MB-231 breast cancer cells: role of autophagy and JNK activation. Cell Death Dis. 4, e638 (2013).
Long, B. H. & Fairchild, C. R. Paclitaxel inhibits progression of mitotic cells to G1 phase by interference with spindle formation without affecting other microtubule functions during anaphase and telophase. Cancer Res. 54, 4355–4361 (1994).
Eum, K. H. & Lee, M. Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol. Cell. Biochem. 348, 61–68 (2011).
Yeatman, T. J. A renaissance for SRC. Nat. Rev. Cancer 4, 470–480 (2004).
Kung, H. J. Targeting tyrosine kinases and autophagy in prostate cancer. Horm. Cancer 2, 38–46 (2011).
Lee, Y. C. et al. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene 29, 3196–3207 (2010).
Wu, Z. et al. Autophagy blockade sensitizes prostate cancer cells towards Src family kinase inhibitors. Genes Cancer 1, 40–49 (2010).
Lamoureux, F. et al. Blocked autophagy using lysosomotropic agents sensitizes resistant prostate tumor cells to the novel Akt inhibitor AZD5363. Clin. Cancer Res. 19, 833–844 (2013).
DaSilva, J., Gioeli, D., Weber, M. J. & Parsons, S. J. The neuroendocrine-derived peptide parathyroid hormone-related protein promotes prostate cancer cell growth by stabilizing the androgen receptor. Cancer Res. 69, 7402–7411 (2009).
Lee, L. F., Guan, J., Qiu, Y. & Kung, H. J. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol. Cell. Biol. 21, 8385–8397 (2001).
Lee, L. F. et al. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene 23, 2197–2205 (2004).
Yang, J. C. et al. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res. 69, 151–160 (2009).
Kim, R. H., Bold, R. J. & Kung, H. J. ADI, autophagy and apoptosis: metabolic stress as a therapeutic option for prostate cancer. Autophagy 5, 567–568 (2009).
Acknowledgements
The work is supported by funding from the following sources: the Department of Defense, the Stand Up To Cancer Prostate Cancer Foundation Prostate Dream Team Translational Cancer Research grant, made possible by the generous support of the Movember Foundation (Stand Up To Cancer is a programme of the Entertainment Industry Foundation administered by the American Association of Cancer Research), and the National Center for Advancing Translational Sciences at the National Institutes of Health, through grant number UL1 TR000002 and the linked TL1 TR000133 award.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, and contributed to discussions of content, and review and editing of the manuscript before submission. J.M.F. wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Farrow, J., Yang, J. & Evans, C. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol 11, 508–516 (2014). https://doi.org/10.1038/nrurol.2014.196
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2014.196
This article is cited by
-
The role of autophagy in prostate cancer and prostatic diseases: a new therapeutic strategy
Prostate Cancer and Prostatic Diseases (2024)
-
Autophagy, a critical element in the aging male reproductive disorders and prostate cancer: a therapeutic point of view
Reproductive Biology and Endocrinology (2023)
-
ATF4/CEMIP/PKCα promotes anoikis resistance by enhancing protective autophagy in prostate cancer cells
Cell Death & Disease (2022)
-
Pre-activation of autophagy impacts response to olaparib in prostate cancer cells
Communications Biology (2022)
-
Autophagy deficiency promotes lung metastasis of prostate cancer via stabilization of TWIST1
Clinical and Translational Oncology (2022)