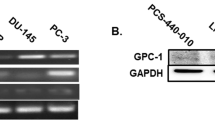
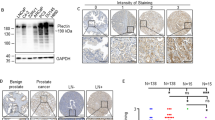
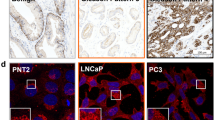
Abstract
The complexity and diversity of proteoglycan structure means that they have a range of functions that regulate cell behavior. Through multiple interactions of their core proteins and glycosaminoglycans with extracellular matrix proteins, growth factors and chemokines, proteoglycans affect cell signaling, motility, adhesion, growth and apoptosis. Progressive changes in proteoglycans occur in the tumor microenvironment, but neither the source nor consequences of those changes are well understood. Proteoglycans studied in prostate cancer include versican—a hyalectan regulator of cell adhesion and migration—and the small leucine-rich proteoglycans decorin, biglycan and lumican, which have roles in cell signaling and tissue organization. Studies support an inhibitory role in prostate cancer for decorin and lumican. Conversely, the basement membrane proteoglycan perlecan might be a tumor promoter through upregulation of sonic hedgehog signaling. Loss of the growth-inhibitory cell-surface proteoglycans syndecan-1 and betaglycan in early prostate cancer might facilitate progression, but syndecan-1 effects are pleiotropic and its renewed expression in advanced tumors might adversely affect outcome. Importantly, cellular changes and enzymatic activity in the developing tumor can alter proteoglycan composition and structure to modify their function. Emerging studies suggest that cancers, including those of the prostate, use these changes to promote their own survival, growth, and spread.
Key Points
-
Proteoglycans are a family of complex macromolecules whose structure comprises a unique core protein substituted with structurally diverse carbohydrate chains termed glycosaminoglycans
-
Both core proteins and glycosaminoglycans participate in interactions with other extracellular matrix proteins, growth factors and chemokines to regulate cell functions
-
Individual proteoglycan species might have opposing roles in regulating cell growth, adhesion, migration and apoptosis
-
The proteoglycan composition of a tissue is the sum of synthetic and degradative activities of multiple cell types and will change in the unstable microenvironment of a developing tumor
-
Proteoglycan fine structure, particularly pattern of glycosaminoglycan sulfation is important in mediating their function; degradative enzymes in a developing tumor might modify that structure and thus alter function
-
In prostate cancer, versican and perlecan promote tumor progression, whereas decorin and betaglycan are tumor suppressors; syndecan-1 might have a dual role as an antagonist and agonist depending on disease stage and enzymatic conditions
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Theocharis, A. D., Skandalis, S. S., Tzanakakis, G. N. & Karamanos, N. K. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 277, 3904–3923 (2010).
Iozzo, R. V. & Schaefer, L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 277, 3864–3875 (2010).
Manon-Jensen, T., Itoh, Y. & Couchman, J. R. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 277, 3876–3889 (2010).
Friedl, A. Proteoglycans: master modulators of paracrine fibroblast-carcinoma cell interactions. Semin. Cell Dev. Biol. 21, 66–71 (2010).
Kjellen, L. & Lindahl, U. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60, 443–475 (1991).
Lindahl, U., Kusche-Gullberg, M. & Kjellen, L. Regulated diversity of heparan sulfate. J. Biol. Chem. 273, 24979–24982 (1998).
Esko, J. D. & Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169–173 (2001).
Gallagher, J. T. Heparan sulfate: growth control with a restricted sequence menu. J. Clin. Invest. 108, 357–361 (2001).
Iozzo, R. V. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 67, 609–652 (1998).
Bernfield, M. et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999).
Thomson, A. A. & Cunha, G. R. Prostatic growth and development are regulated by FGF10. Development 126, 3693–3701 (1999).
Mohammadi, M., Olsen, S. K. & Ibrahimi, O. A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 16, 107–137 (2005).
Buresh, R. A. et al. Sulfatase 1 is an inhibitor of ductal morphogenesis with sexually dimorphic expression in the urogenital sinus. Endocrinology 151, 3420–3431 (2010).
Podlasek, C. A., Barnett, D. H., Clemens, J. Q., Bak, P. M. & Bushman, W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev. Biol. 209, 28–39 (1999).
Datta, S., Pierce, M. & Datta, M. W. Perlecan signaling: helping hedgehog stimulate prostate cancer growth. Int. J. Biochem. Cell Biol. 38, 1855–1861 (2006).
Sakko, A. J. et al. Changes in steroid receptors and proteoglycan expression in the guinea pig prostate stroma during puberty and hormone manipulation. Prostate 67, 288–300 (2007).
Kolset, S. O. & Tveit, H. Serglycin--structure and biology. Cell. Mol. Life Sci. 65, 1073–1085 (2008).
Niemann, C. U., Kjeldsen, L., Ralfkiaer, E., Jensen, M. K. & Borregaard, N. Serglycin proteoglycan in hematologic malignancies: a marker of acute myeloid leukemia. Leukemia 21, 2406–2410 (2007).
Meen, A. J. et al. Serglycin is a major proteoglycan in polarized human endothelial cells and is implicated in the secretion of the chemokine GROalpha/CXCL1. J. Biol. Chem. 286, 2636–2647 (2011).
Li, X. J. et al. Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes metastasis. Cancer Res. 71, 3162–3172 (2011).
Theocharis, A. D. et al. Serglycin constitutively secreted by myeloma plasma cells is a potent inhibitor of bone mineralization in vitro. J. Biol. Chem. 281, 35116–35128 (2006).
Cohen, R. J., Holland, J. W., Redmond, S. L., McNeal, J. E. & Dawkins, H. J. Identification of the glycosaminoglycan keratan sulfate in the prostatic secretory cell. Prostate 44, 204–209 (2000).
Holland, J. W., Meehan, K. L., Redmond, S. L. & Dawkins, H. J. Purification of the keratan sulfate proteoglycan expressed in prostatic secretory cells and its identification as lumican. Prostate 59, 252–259 (2004).
Ricciardelli, C., Sakko, A. J., Ween, M. P., Russell, D. L. & Horsfall, D. J. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 28, 233–245 (2009).
Wight, T. N. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 14, 617–623 (2002).
Wu, Y. J., La Pierre, D. P., Wu, J., Yee, A. J. & Yang, B. B. The interaction of versican with its binding partners. Cell Res. 15, 483–494 (2005).
Hirose, J., Kawashima, H., Yoshie, O., Tashiro, K. & Miyasaka, M. Versican interacts with chemokines and modulates cellular responses. J. Biol. Chem. 276, 5228–5234 (2001).
Dours-Zimmermann, M. T. & Zimmermann, D. R. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J. Biol. Chem. 269, 32992–32998 (1994).
Sakko, A. J. et al. Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res. 63, 4786–4791 (2003).
Ricciardelli, C. et al. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin. Cancer Res. 4, 963–971 (1998).
Ricciardelli, C. et al. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J. Biol. Chem. 282, 10814–10825 (2007).
Read, J. T. et al. Androgen receptor regulation of the versican gene through an androgen response element in the proximal promoter. J. Biol. Chem. 282, 31954–31963 (2007).
Sakko, A. J. et al. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res. 61, 926–930 (2001).
Cross, N. A. et al. The expression and regulation of ADAMTS-1, -4, -5, -9, and -15, and TIMP-3 by TGFbeta1 in prostate cells: relevance to the accumulation of versican. Prostate 63, 269–275 (2005).
Sandy, J. D. et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J. Biol. Chem. 276, 13372–13378 (2001).
Somerville, R. P. et al. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J. Biol. Chem. 278, 9503–9513 (2003).
Schaefer, L. & Iozzo, R. V. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J. Biol. Chem. 283, 21305–21309 (2008).
Scott, P. G. et al. Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan. Proc. Natl Acad. Sci. USA 101, 15633–15638 (2004).
Schaefer, L. & Schaefer, R. M. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 339, 237–246 (2010).
Weber, I. T., Harrison, R. W. & Iozzo, R. V. Model structure of decorin and implications for collagen fibrillogenesis. J. Biol. Chem. 271, 31767–31770 (1996).
Neame, P. J., Kay, C. J., McQuillan, D. J., Beales, M. P. & Hassell, J. R. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell. Mol. Life Sci. 57, 859–863 (2000).
Svensson, L. et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J. Biol. Chem. 274, 9636–9647 (1999).
Yamaguchi, Y., Mann, D. M. & Ruoslahti, E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 346, 281–284 (1990).
Moscatello, D. K. et al. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J. Clin. Invest. 101, 406–412 (1998).
Iozzo, R. V., Moscatello, D. K., McQuillan, D. J. & Eichstetter, I. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 274, 4489–4492 (1999).
Santra, M., Eichstetter, I. & Iozzo, R. V. An anti-oncogenic role for decorin. Downregulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J. Biol. Chem. 275, 35153–35161 (2000).
Csordas, G. et al. Sustained downregulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 275, 32879–32887 (2000).
Goldoni, S. et al. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 185, 743–754 (2009).
Schonherr, E., Sunderkotter, C., Iozzo, R. V. & Schaefer, L. Decorin, a novel player in the insulin-like growth factor system. J. Biol. Chem. 280, 15767–15772 (2005).
Mauviel, A., Santra, M., Chen, Y. Q., Uitto, J. & Iozzo, R. V. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-alpha. J. Biol. Chem. 270, 11692–11700 (1995).
Santra, M. et al. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J. Clin. Invest. 100, 149–157 (1997).
Reed, C. C., Gauldie, J. & Iozzo, R. V. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene 21, 3688–3695 (2002).
Reed, C. C. et al. Decorin prevents metastatic spreading of breast cancer. Oncogene 24, 1104–1110 (2005).
Biglari, A. et al. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Ther. 11, 721–732 (2004).
Tralhao, J. G. et al. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer. FASEB J. 17, 464–466 (2003).
Banerjee, A. G. et al. Expression of biomarkers modulating prostate cancer angiogenesis: differential expression of annexin II in prostate carcinomas from India and USA. Mol. Cancer 2, 34 (2003).
Coulson-Thomas, V. J. et al. Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-beta, and extracellular matrix downregulation. Exp. Cell Res. 316, 3207–3226 (2010).
Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003).
Hu, Y. et al. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia 11, 1042–1053 (2009).
Mimeault, M. & Batra, S. K. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis 27, 1–22 (2006).
Fassan, M. et al. Mitostatin is downregulated in human prostate cancer and suppresses the invasive phenotype of prostate cancer cells. PLoS ONE 6, e19771 (2011).
Vecchione, A. et al. MITOSTATIN, a putative tumor suppressor on chromosome 12q24.1, is downregulated in human bladder and breast cancer. Oncogene 28, 257–269 (2009).
Chen, N. et al. A secreted isoform of ErbB3 promotes osteonectin expression in bone and enhances the invasiveness of prostate cancer cells. Cancer Res. 67, 6544–6548 (2007).
Chen, X. D., Fisher, L. W., Robey, P. G. & Young, M. F. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 18, 948–958 (2004).
Wang, J., Levenson, A. S. & Satcher, R. L. Jr. Identification of a unique set of genes altered during cell-cell contact in an in vitro model of prostate cancer bone metastasis. Int. J. Mol. Med. 17, 849–856 (2006).
Chakravarti, S. et al. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J. Cell Biol. 141, 1277–1286 (1998).
Kao, W. W. & Liu, C. Y. Roles of lumican and keratocan on corneal transparency. Glycoconj. J. 19, 275–285 (2002).
Leygue, E. et al. Expression of lumican in human breast carcinoma. Cancer Res. 58, 1348–1352 (1998).
Matsuda, Y. et al. Expression and roles of lumican in lung adenocarcinoma and squamous cell carcinoma. Int. J. Oncol. 33, 1177–1185 (2008).
Ishiwata, T. et al. Role of lumican in cancer cells and adjacent stromal tissues in human pancreatic cancer. Oncol. Rep. 18, 537–543 (2007).
Brezillon, S. et al. Expression of lumican, a small leucine-rich proteoglycan with antitumor activity, in human malignant melanoma. Clin. Exp. Dermatol. 32, 405–416 (2007).
D'Onofrio, M. F. et al. Identification of beta1 integrin as mediator of melanoma cell adhesion to lumican. Biochem. Biophys. Res. Commun. 365, 266–272 (2008).
Brezillon, S. et al. Lumican inhibits B16F1 melanoma cell lung metastasis. J. Physiol. Pharmacol. 60 (Suppl. 4), 15–22 (2009).
Vij, N., Roberts, L., Joyce, S. & Chakravarti, S. Lumican suppresses cell proliferation and aids Fas-Fas ligand mediated apoptosis: implications in the cornea. Exp. Eye Res. 78, 957–971 (2004).
Lee, S., Bowrin, K., Hamad, A. R. & Chakravarti, S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to beta2 integrin. J. Biol. Chem. 284, 23662–23669 (2009).
Leygue, E. et al. Lumican and decorin are differentially expressed in human breast carcinoma. J. Pathol. 192, 313–320 (2000).
Luo, J. et al. Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate 51, 189–200 (2002).
Iozzo, R. V., Zoeller, J. J. & Nystrom, A. Basement membrane proteoglycans: modulators Par Excellence of cancer growth and angiogenesis. Mol. Cells 27, 503–513 (2009).
Iozzo, R. V. & San Antonio, J. D. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J. Clin. Invest. 108, 349–355 (2001).
Murdoch, A. D., Liu, B., Schwarting, R., Tuan, R. S. & Iozzo, R. V. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J. Histochem. Cytochem. 42, 239–249 (1994).
Iozzo, R. V., Cohen, I. R., Grassel, S. & Murdoch, A. D. The biology of perlecan: the multifaceted heparan sulfate proteoglycan of basement membranes and pericellular matrices. Biochem. J. 302 (Pt 3), 625–639 (1994).
Savore, C. et al. Perlecan knockdown in metastatic prostate cancer cells reduces heparin-binding growth factor responses in vitro and tumor growth in vivo. Clin. Exp. Metastasis 22, 377–390 (2005).
Datta, M. W. et al. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol. Cancer 5, 9 (2006).
Karhadkar, S. S. et al. Hedgehog signaling in prostate regeneration, neoplasia and metastasis. Nature 431, 707–712 (2004).
Gatza, C. E., Oh, S. Y. & Blobe, G. C. Roles for the type III TGF-beta receptor in human cancer. Cell Signal 22, 1163–1174 (2010).
Bernfield, M. et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8, 365–393 (1992).
Kim, C. W., Goldberger, O. A., Gallo, R. L. & Bernfield, M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 5, 797–805 (1994).
Liu, W. et al. Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J. Biol. Chem. 273, 22825–22832 (1998).
Woods, A., Oh, E. S. & Couchman, J. R. Syndecan proteoglycans and cell adhesion. Matrix Biol. 17, 477–483 (1998).
Liebersbach, B. F. & Sanderson, R. D. Expression of syndecan-1 inhibits cell invasion into type I collagen. J. Biol. Chem. 269, 20013–20019 (1994).
Dhodapkar, M. V. et al. Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood 91, 2679–2688 (1998).
Endo, K. et al. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 278, 40764–40770 (2003).
Kato, M. et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat. Med. 4, 691–697 (1998).
Mali, M., Elenius, K., Miettinen, H. M. & Jalkanen, M. Inhibition of basic fibroblast growth factor-induced growth promotion by overexpression of syndecan-1. J. Biol. Chem. 268, 24215–24222 (1993).
Mali, M., Andtfolk, H., Miettinen, H. M. & Jalkanen, M. Suppression of tumor cell growth by syndecan-1 ectodomain. J. Biol. Chem. 269, 27795–27798 (1994).
Nikolova, V. et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis 30, 397–407 (2009).
Inki, P., Joensuu, H., Grenman, R., Klemi, P. & Jalkanen, M. Association between syndecan-1 expression and clinical outcome in squamous cell carcinoma of the head and neck. Br. J. Cancer 70, 319–323 (1994).
Matsumoto, A. et al. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int. J. Cancer 74, 482–491 (1997).
Nackaerts, K. et al. Heparan sulfate proteoglycan expression in human lung-cancer cells. Int. J. Cancer 74, 335–345 (1997).
Pulkkinen, J. O., Penttinen, M., Jalkanen, M., Klemi, P. & Grenman, R. Syndecan-1: a new prognostic marker in laryngeal cancer. Acta Otolaryngol. 117, 312–315 (1997).
Anttonen, A., Kajanti, M., Heikkila, P., Jalkanen, M. & Joensuu, H. Syndecan-1 expression has prognostic significance in head and neck carcinoma. Br. J. Cancer 79, 558–564 (1999).
Numa, F. et al. Syndecan-1 expression in cancer of the uterine cervix: association with lymph node metastasis. Int. J. Oncol. 20, 39–43 (2002).
Loussouarn, D. et al. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. Br. J. Cancer 98, 1993–1998 (2008).
Leppa, S., Mali, M., Miettinen, H. M. & Jalkanen, M. Syndecan expression regulates cell morphology and growth of mouse mammary epithelial tumor cells. Proc. Natl Acad. Sci. USA 89, 932–936 (1992).
Sun, H., Berquin, I. M., Owens, R. T., O'Flaherty, J. T. & Edwards, I. J. Peroxisome proliferator-activated receptor gamma-mediated upregulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 68, 2912–2919 (2008).
Hu, Y. et al. Syndecan-1-dependent suppression of PDK1/Akt/bad signaling by docosahexaenoic acid induces apoptosis in prostate cancer. Neoplasia 12, 826–836 (2010).
Conejo, J. R. et al. Syndecan-1 expression is upregulated in pancreatic but not in other gastrointestinal cancers. Int. J. Cancer 88, 12–20 (2000).
Barbareschi, M. et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer 98, 474–483 (2003).
Davies, E. J. et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin. Cancer Res. 10, 5178–5186 (2004).
Watanabe, A. et al. Expression of syndecans, a heparan sulfate proteoglycan, in malignant gliomas: participation of nuclear factor-kappaB in upregulation of syndecan-1 expression. J. Neurooncol. 77, 25–32 (2006).
Choi, D. S. et al. Syndecan-1, a key regulator of cell viability in endometrial cancer. Int. J. Cancer 121, 741–750 (2007).
Mennerich, D. et al. Shift of syndecan-1 expression from epithelial to stromal cells during progression of solid tumors. Eur. J. Cancer 40, 1373–1382 (2004).
Stanley, M. J., Stanley, M. W., Sanderson, R. D. & Zera, R. Syndecan-1 expression is induced in the stroma of infiltrating breast carcinoma. Am. J. Clin. Pathol. 112, 377–383 (1999).
Maeda, T., Alexander, C. M. & Friedl, A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 64, 612–621 (2004).
Maeda, T., Desouky, J. & Friedl, A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene 25, 1408–1412 (2006).
Su, G., Blaine, S. A., Qiao, D. & Friedl, A. Shedding of syndecan-1 by stromal fibroblasts stimulates human breast cancer cell proliferation via FGF2 activation. J. Biol. Chem. 282, 14906–14915 (2007).
Sanderson, R. D. et al. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J. Cell Biochem. 96, 897–905 (2005).
Chen, D. et al. Syndecan-1 expression in locally invasive and metastatic prostate cancer. Urology 63, 402–407 (2004).
Kiviniemi, J. et al. Altered expression of syndecan-1 in prostate cancer. APMIS 112, 89–97 (2004).
Brimo, F., Vollmer, R. T., Friszt, M., Corcos, J. & Bismar, T. A. Syndecan-1 expression in prostate cancer and its value as biomarker for disease progression. BJU Int. 106, 418–423 (2010).
Shariat, S. F. et al. Prognostic value of syndecan-1 expression in patients treated with radical prostatectomy. BJU Int. 101, 232–237 (2008).
Zellweger, T. et al. Expression patterns of potential therapeutic targets in prostate cancer. Int. J. Cancer 113, 619–628 (2005).
Lerner, I. et al. Function of heparanase in prostate tumorigenesis: potential for therapy. Clin. Cancer Res. 14, 668–676 (2008).
Edwards, I. J. et al. In vivo and in vitro regulation of syndecan 1 in prostate cells by N.-3 polyunsaturated fatty acids. J. Biol. Chem. 283, 18441–18449 (2008).
Danielpour, D. Functions and regulation of transforming growth factor-beta (TGF-beta) in the prostate. Eur. J. Cancer 41, 846–857 (2005).
Sharifi, N., Hurt, E. M., Kawasaki, B. T. & Farrar, W. L. TGFBR3 loss and consequences in prostate cancer. Prostate 67, 301–311 (2007).
Ajiboye, S., Sissung, T. M., Sharifi, N. & Figg, W. D. More than an accessory: implications of type III transforming growth factor-beta receptor loss in prostate cancer. BJU Int. 105, 913–916 (2010).
Turley, R. S. et al. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 67, 1090–1098 (2007).
Lopez-Casillas, F., Payne, H. M., Andres, J. L. & Massague, J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J. Cell Biol. 124, 557–568 (1994).
Bandyopadhyay, A. et al. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. Prostate 63, 81–90 (2005).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Edwards, I. Proteoglycans in prostate cancer. Nat Rev Urol 9, 196–206 (2012). https://doi.org/10.1038/nrurol.2012.19
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2012.19
This article is cited by
-
Inter- and intratumoral proteomics and glycosaminoglycan characterization of ALK rearranged lung adenocarcinoma tissues: a pilot study
Scientific Reports (2023)
-
MiRNA-30a-5p/VCAN Arrests Tumor Metastasis via Modulating the Adhesion of Lung Adenocarcinoma Cells
Applied Biochemistry and Biotechnology (2023)
-
Spatio-temporal analysis of prostate tumors in situ suggests pre-existence of treatment-resistant clones
Nature Communications (2022)
-
Identification of hub genes predicting the development of prostate cancer from benign prostate hyperplasia and analyzing their clinical value in prostate cancer by bioinformatic analysis
Discover Oncology (2022)
-
Bioinformatics Prediction and Analysis of MicroRNAs and Their Targets as Biomarkers for Prostate Cancer: A Preliminary Study
Molecular Biotechnology (2022)