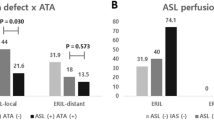
Abstract
Brain imaging provides an objective basis for the clinical inferences that direct individual patient management in the acute stroke setting. A brain CT or MRI scan is required for all patients with suspected stroke or transient ischemic attack. Thrombolytic therapy is arguably the most important aspect of acute stroke management; however, most decisions in acute stroke do not relate to this treatment. Stroke imaging must, therefore, provide information beyond the presence or absence of intracranial hemorrhage (ICH) and early evidence of a large infarct. Noncontrast CT and gradient-recalled echo MRI show comparable accuracy in the diagnosis of acute ICH. Diffusion-weighted MRI is more sensitive than noncontrast CT for differentiation of acute ischemic stroke from nonstroke conditions. Combined multimodal parenchymal, perfusion and vascular imaging with CT or MRI has the potential to identify patients with an ischemic penumbra that might be appropriate for acute reperfusion therapies. MRI identifies a broader range of acute and chronic cerebrovascular pathologies than does CT and, hence, could aid decisions about acute intervention, in-hospital management, and secondary prevention. Here, we present an overview of the diagnostic information that clinicians might gain from CT and MRI in the setting of acute stroke, along with the advantages and disadvantages of these techniques.
Key Points
-
A brain CT or MRI scan is urgently recommended for all patients with suspected acute stroke or transient ischemic attack
-
Noncontrast CT and gradient-recalled echo MRI show similar accuracy in the diagnosis of acute intracerebral hemorrhage
-
Diffusion-weighted MRI is markedly more sensitive than noncontrast CT for distinguishing acute ischemic stroke from nonstroke conditions
-
Combined multimodal parenchymal, perfusion and vascular imaging with CT or MRI could potentially identify patients with an ischemic penumbra that might be amenable to acute reperfusion therapies
-
MRI identifies a broad range of acute and chronic cerebrovascular pathologies that could aid decisions about acute intervention, in-hospital management and secondary prevention
-
Overall, MRI is diagnostically superior to CT for cerebrovascular indications, but is contraindicated in ≈10% of patients, has limited availability at many hospitals, and can be costly and time-consuming
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Latchaw, R. E. et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 40, 3646–3678 (2009).
Ringleb, P. A. et al. Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. The European Stroke Organisation (ESO) [online] (2008).
Baird, A. E. & Warach, S. Magnetic resonance imaging of acute stroke. J. Cereb. Blood Flow Metab. 18, 583–609 (1998).
Ledezma, C. J. & Wintermark, M. Multimodal CT in stroke imaging: new concepts. Radiol. Clin. North Am. 47, 109–116 (2009).
Kang, D. W., Latour, L. L., Chalela, J. A., Dambrosia, J. & Warach, S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann. Neurol. 54, 66–74 (2003).
Kang, D. W., Latour, L. L., Chalela, J. A., Dambrosia, J. A. & Warach, S. Early and late recurrence of ischemic lesion on MRI: evidence for a prolonged stroke-prone state? Neurology 63, 2261–2265 (2004).
Latour, L. L., Kang, D. W., Ezzeddine, M. A., Chalela, J. A. & Warach, S. Early blood–brain barrier disruption in human focal brain ischemia. Ann. Neurol. 56, 468–477 (2004).
Hacke, W. et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 36, 66–73 (2005).
Albers, G. W. et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann. Neurol. 60, 508–517 (2006).
Davis, S. M. et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 7, 299–309 (2008).
Chalela, J. A. et al. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: Insights into the pathophysiology of acute stroke in the thrombolysis era. Ann. Neurol. 55, 105–112 (2004).
Merino, J. G. et al. Lesion volume change after treatment with tissue plasminogen activator can discriminate clinical responders from nonresponders. Stroke 38, 2919–2923 (2007).
Merino, J. G. et al. Reperfusion half-life: a novel pharmacodynamic measure of thrombolytic activity. Stroke 39, 2148–2150 (2008).
Sharma, V. K., Tsivgoulis, G., Lao, A. Y. & Alexandrov, A. V. Role of transcranial Doppler ultrasonography in evaluation of patients with cerebrovascular disease. Curr. Neurol. Neurosci. Rep. 7, 8–20 (2007).
Tsivgoulis, G., Alexandrov, A. V. & Sloan, M. A. Advances in transcranial Doppler ultrasonography. Curr. Neurol. Neurosci. Rep. 9, 46–54 (2009).
Kucinski, T., Koch, C. & Zeumer, H. In Imaging in Stroke (ed. Hennerici, M. G.) 19–42 (Remedica Publishing, London, 2003).
Schriger, D. L., Kalafut, M., Starkman, S., Krueger, M. & Saver, J. L. Cranial computed tomography interpretation in acute stroke: physician accuracy in determining eligibility for thrombolytic therapy. JAMA 279, 1293–1297 (1998).
Sames, T. A., Storrow, A. B., Finkelstein, J. A. & Magoon, M. R. Sensitivity of new-generation computed tomography in subarachnoid hemorrhage. Acad. Emerg. Med. 3, 16–20 (1996).
Bederson, J. B. et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 40, 994–1025 (2009).
Adams, H. P. Jr et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38, 1655–1711 (2007).
Simard, J. M., Kent, T. A., Chen, M., Tarasov, K. V. & Gerzanich, V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 6, 258–268 (2007).
Tomura, N. et al. Early CT finding in cerebral infarction: obscuration of the lentiform nucleus. Radiology 168, 463–467 (1988).
Truwit, C. L., Barkovich, A. J., Gean-Marton, A., Hibri, N. & Norman, D. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology 176, 801–806 (1990).
von Kummer, R. et al. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology 205, 327–333 (1997).
Marks, M. P. et al. Evaluation of early computed tomographic findings in acute ischemic stroke. Stroke 30, 389–392 (1999).
Patel, S. C. et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA 286, 2830–2838 (2001).
von Kummer, R. et al. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology 219, 95–100 (2001).
Larrue, V., von Kummer, R. R., Muller, A. & Bluhmki, E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 32, 438–441 (2001).
Barber, P. A., Demchuk, A. M., Zhang, J. & Buchan, A. M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 355, 1670–1674 (2000).
Lev, M. H. et al. Acute stroke: improved nonenhanced CT detection—benefits of soft-copy interpretation by using variable window width and center level settings. Radiology 213, 150–155 (1999).
Na, D. G. et al. CT sign of brain swelling without concomitant parenchymal hypoattenuation: comparison with diffusion- and perfusion-weighted MR imaging. Radiology 235, 992–948 [sic; 992–998] (2005).
Gacs, G., Fox, A. J., Barnett, H. J. & Vinuela, F. CT visualization of intracranial arterial thromboembolism. Stroke 14, 756–762 (1983).
Leys, D., Pruvo, J. P., Godefroy, O., Rondepierre, P. & Leclerc, X. Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke 23, 317–324 (1992).
Krings, T. et al. The hyperdense posterior cerebral artery sign: a computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke 37, 399–403 (2006).
Goldmakher, G. V. et al. Hyperdense basilar artery sign on unenhanced CT predicts thrombus and outcome in acute posterior circulation stroke. Stroke 40, 134–139 (2009).
Barber, P. A. et al. Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemia. Stroke 32, 84–88 (2001).
von Kummer, R. et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am. J. Neuroradiol. 15, 9–18 (1994).
Kharitonova, T. et al. Disappearing hyperdense middle cerebral artery sign in ischaemic stroke patients treated with intravenous thrombolysis: clinical course and prognostic significance. J. Neurol. Neurosurg. Psychiatry 80, 273–278 (2009).
Lev, M. H. et al. Total occlusion versus hairline residual lumen of the internal carotid arteries: accuracy of single section helical CT angiography. AJNR Am. J. Neuroradiol. 24, 1123–1129 (2003).
Lubezky, N., Fajer, S., Barmeir, E. & Karmeli, R. Duplex scanning and CT angiography in the diagnosis of carotid artery occlusion: a prospective study. Eur. J. Vasc. Endovasc. Surg. 16, 133–136 (1998).
Lev, M. H. et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J. Comput. Assist. Tomogr. 25, 520–528 (2001).
Lev, M. H. et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 32, 2021–2028 (2001).
Hirai, T. et al. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. AJNR Am. J. Neuroradiol. 23, 93–101 (2002).
Skutta, B., Furst, G., Eilers, J., Ferbert, A. & Kuhn, F. P. Intracranial stenoocclusive disease: double-detector helical CT angiography versus digital subtraction angiography. AJNR Am. J. Neuroradiol. 20, 791–799 (1999).
Bash, S. et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am. J. Neuroradiol. 26, 1012–1021 (2005).
Ezzeddine, M. A. et al. CT angiography with whole brain perfused blood volume imaging: added clinical value in the assessment of acute stroke. Stroke 33, 959–966 (2002).
Schramm, P. et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke 35, 1652–1658 (2004).
Hunter, G. J. et al. Assessment of cerebral perfusion and arterial anatomy in hyperacute stroke with three-dimensional functional CT: early clinical results. AJNR Am. J. Neuroradiol. 19, 29–37 (1998).
Papke, K. et al. Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning. Radiology 244, 532–540 (2007).
Villablanca, J. P. et al. Volume-rendered helical computerized tomography angiography in the detection and characterization of intracranial aneurysms. J. Neurosurg. 93, 254–264 (2000).
Wardlaw, J. M. & White, P. M. The detection and management of unruptured intracranial aneurysms. Brain 123, 205–221 (2000).
Chaudhary, S. R. et al. Prospective evaluation of multidetector-row CT angiography for the diagnosis of vasospasm following subarachnoid hemorrhage: a comparison with digital subtraction angiography. Cerebrovasc. Dis. 25, 144–150 (2008).
Wintermark, M. et al. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke 33, 2025–2031 (2002).
Wintermark, M. et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann. Neurol. 51, 417–432 (2002).
Parsons, M. W. Perfusion CT: is it clinically useful? Int. J. Stroke 3, 41–50 (2008).
Wintermark, M. et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 37, 979–985 (2006).
Kang, D. W., Chalela, J. A., Dunn, W. & Warach, S. MRI screening before standard tissue plasminogen activator therapy is feasible and safe. Stroke 36, 1939–1943 (2005).
Schellinger, P. D. et al. Feasibility and practicality of MR imaging of stroke in the management of hyperacute cerebral ischemia. AJNR Am. J. Neuroradiol. 21, 1184–1189 (2000).
Kohrmann, M. et al. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol. 5, 661–667 (2006).
Schellinger, P. D. et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke 38, 2640–2645 (2007).
Earnshaw, S. R., Jackson, D., Farkouh, R. & Schwamm, L. Cost-effectiveness of patient selection using penumbral-based MRI for intravenous thrombolysis. Stroke 40, 1710–1720 (2009).
Hjort, N. et al. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke 36, 388–397 (2005).
Warach, S., Chien, D., Li, W., Ronthal, M. & Edelman, R. R. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology 42, 1717–1723 (1992).
Warach, S., Gaa, J., Siewert, B., Wielopolski, P. & Edelman, R. R. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann. Neurol. 37, 231–241 (1995).
Schlaug, G., Siewert, B., Benfield, A., Edelman, R. R. & Warach, S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 49, 113–119 (1997).
Chalela, J. A. et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 369, 293–298 (2007).
Sylaja, P. N., Coutts, S. B., Krol, A., Hill, M. D. & Demchuk, A. M. When to expect negative diffusion-weighted images in stroke and transient ischemic attack. Stroke 39, 1898–1900 (2008).
Barber, P. A. et al. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke 30, 2059–2065 (1999).
Lansberg, M. G., Albers, G. W., Beaulieu, C. & Marks, M. P. Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology 54, 1557–1561 (2000).
Baird, A. E. et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann. Neurol. 41, 581–589 (1997).
Beaulieu, C. et al. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann. Neurol. 46, 568–578 (1999).
Lansberg, M. G., O'Brien, M. W., Tong, D. C., Moseley, M. E. & Albers, G. W. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch. Neurol. 58, 613–617 (2001).
Schwamm, L. H. et al. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke 29, 2268–2276 (1998).
Kidwell, C. S. et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann. Neurol. 47, 462–469 (2000).
Fiehler, J. et al. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke 33, 79–86 (2002).
Schlaug, G. et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 53, 1528–1537 (1999).
Jansen, O., Schellinger, P., Fiebach, J., Hacke, W. & Sartor, K. Early recanalisation in acute ischaemic stroke saves tissue at risk defined by MRI. Lancet 353, 2036–2037 (1999).
Parsons, M. W. et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann. Neurol. 51, 28–37 (2002).
Bykowski, J. L., Latour, L. L. & Warach, S. More accurate identification of reversible ischemic injury in human stroke by cerebrospinal fluid suppressed diffusion-weighted imaging. Stroke 35, 1100–1106 (2004).
Kidwell, C. S., Alger, J. R. & Saver, J. L. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke 34, 2729–2735 (2003).
Warach, S., Dashe, J. F. & Edelman, R. R. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J. Cereb. Blood Flow Metab. 16, 53–59 (1996).
Lovblad, K. O. et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann. Neurol. 42, 164–170 (1997).
Barber, P. A. et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 51, 418–426 (1998).
Baird, A. E. et al. A three-item scale for the early prediction of stroke recovery. Lancet 357, 2095–2099 (2001).
Sylaja, P. N. et al. Acute ischemic lesions of varying ages predict risk of ischemic events in stroke/TIA patients. Neurology 68, 415–419 (2007).
Kang, D. W., Chalela, J. A., Ezzeddine, M. A. & Warach, S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch. Neurol. 60, 1730–1734 (2003).
Baird, A. E., Lovblad, K. O., Schlaug, G., Edelman, R. R. & Warach, S. Multiple acute stroke syndrome: marker of embolic disease? Neurology 54, 674–678 (2000).
Chaves, C. J. et al. Diffusion- and perfusion-weighted MRI patterns in borderzone infarcts. Stroke 31, 1090–1096 (2000).
Kidwell, C. S. et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 30, 1174–1180 (1999).
Easton, J. D. et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 40, 2276–2293 (2009).
Prabhakaran, S., Chong, J. Y. & Sacco, R. L. Impact of abnormal diffusion-weighted imaging results on short-term outcome following transient ischemic attack. Arch. Neurol. 64, 1105–1109 (2007).
Coutts, S. B. et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann. Neurol. 57, 848–854 (2005).
Purroy, F. et al. Higher risk of further vascular events among transient ischemic attack patients with diffusion-weighted imaging acute ischemic lesions. Stroke 35, 2313–2319 (2004).
Albers, G. W. et al. Transient ischemic attack—proposal for a new definition. N. Engl. J. Med. 347, 1713–1716 (2002).
Haacke, E. M., Mittal, S., Wu, Z., Neelavalli, J. & Cheng, Y. C. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am. J. Neuroradiol. 30, 19–30 (2009).
Linfante, I., Llinas, R. H., Caplan, L. R. & Warach, S. MRI features of intracerebral hemorrhage within 2 hours from symptom onset. Stroke 30, 2263–2267 (1999).
Kidwell, C. S. et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 292, 1823–1830 (2004).
Edelman, R. R. et al. MR of hemorrhage: a new approach. AJNR Am. J. Neuroradiol. 7, 751–756 (1986).
Patel, M. R., Edelman, R. R. & Warach, S. Detection of hyperacute primary intraparenchymal hemorrhage by magnetic resonance imaging. Stroke 27, 2321–2324 (1996).
Atlas, S. W. & Thulborn, K. R. MR detection of hyperacute parenchymal hemorrhage of the brain. AJNR Am. J. Neuroradiol. 19, 1471–1477 (1998).
Fiebach, J. B. et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke 35, 502–506 (2004).
Fazekas, F. et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am. J. Neuroradiol. 20, 637–642 (1999).
Greenberg, S. M. et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 8, 165–174 (2009).
Knudsen, K. A., Rosand, J., Karluk, D. & Greenberg, S. M. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 56, 537–539 (2001).
Klein, I. et al. Cerebral microbleeds are frequent in infective endocarditis: a case–control study. Stroke 40, 3461–3465 (2009).
Nighoghossian, N. et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke 33, 735–742 (2002).
Kato, H., Izumiyama, M., Izumiyama, K., Takahashi, A. & Itoyama, Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke 33, 1536–1540 (2002).
Fiehler, J. et al. MR STROKE Group. Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke 38, 2738–2744 (2007).
McCarron, M. O. & Nicoll, J. A. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 3, 484–492 (2004).
Greenberg, S. M., Eng, J. A., Ning, M., Smith, E. E. & Rosand, J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 35, 1415–1420 (2004).
Boulanger, J. M. et al. Cerebral microhemorrhages predict new disabling or fatal strokes in patients with acute ischemic stroke or transient ischemic attack. Stroke 37, 911–914 (2006).
Chalela, J. A., Haymore, J. B., Ezzeddine, M. A., Davis, L. A. & Warach, S. The hypointense MCA sign. Neurology 58, 1470 (2002).
Cho, K. H., Kim, J. S., Kwon, S. U., Cho, A. H. & Kang, D. W. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke 36, 2379–2383 (2005).
Mitchell, P. et al. Detection of subarachnoid haemorrhage with magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 70, 205–211 (2001).
Kidwell, C. S. et al. Thrombolytic toxicity: blood–brain barrier disruption in human ischemic stroke. Cerebrovasc. Dis. 25, 338–343 (2008).
Warach, S. & Latour, L. L. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood–brain barrier disruption. Stroke 35 (11 Suppl. 1), 2659–2661 (2004).
Barr, T. L. et al. Blood–brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 41, e123–e128 (2009).
Cho, A. H. et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovasc. Dis. 25, 572–579 (2008).
Ebinger, M. et al. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke 41, 250–255 (2010).
Song, S. S., Ritter, C. H., Ku, K. D., Latour, L. L. & Warach, S. The upper time limit of DWI positive–FLAIR negative MRI in witnessed-onset acute ischemic strokes is less than 6 hours: implications for the design of wake-up stroke treatment trials. Stroke 41, e48 (2010).
Thomalla, G. et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann. Neurol. 65, 724–732 (2009).
Kamran, S. et al. Significance of hyperintense vessels on FLAIR MRI in acute stroke. Neurology 55, 265–269 (2000).
Toyoda, K., Ida, M. & Fukuda, K. Fluid-attenuated inversion recovery intraarterial signal: an early sign of hyperacute cerebral ischemia. AJNR Am. J. Neuroradiol. 22, 1021–1029 (2001).
Schellinger, P. D., Chalela, J. A., Kang, D. W., Latour, L. L. & Warach, S. Diagnostic and prognostic value of early MR Imaging vessel signs in hyperacute stroke patients imaged <3 hours and treated with recombinant tissue plasminogen activator. AJNR Am. J. Neuroradiol. 26, 618–624 (2005).
Lee, K. Y. et al. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology 72, 1134–1139 (2009).
Debrey, S. M. et al. Diagnostic accuracy of magnetic resonance angiography for internal carotid artery disease: a systematic review and meta-analysis. Stroke 39, 2237–2248 (2008).
Raghavan, P., Mukherjee, S., Gaughen, J. & Phillips, C. D. Magnetic resonance angiography of the extracranial carotid system. Top. Magn. Reson. Imaging 19, 241–249 (2008).
Babiarz, L. S. et al. Contrast-enhanced MR angiography is not more accurate than unenhanced 2D time-of-flight MR angiography for determining ≥70% internal carotid artery stenosis. AJNR Am. J. Neuroradiol. 30, 761–768 (2009).
Provenzale, J. M. & Sarikaya, B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am. J. Roentgenol. 193, 1167–1174 (2009).
Korogi, Y. et al. Intracranial aneurysms: diagnostic accuracy of MR angiography with evaluation of maximum intensity projection and source images. Radiology 199, 199–207 (1996).
Rosen, B. R., Belliveau, J. W., Vevea, J. M. & Brady, T. J. Perfusion imaging with NMR contrast agents. Magn. Reson. Med. 14, 249–265 (1990).
Christensen, S. et al. Comparison of 10 perfusion MRI parameters in 97 sub-6-hour stroke patients using voxel-based receiver operating characteristics analysis. Stroke 40, 2055–2061 (2009).
Kane, I. et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke 38, 3158–3164 (2007).
Warach, S. Thrombolysis in stroke beyond three hours: targeting patients with diffusion and perfusion MRI. Ann. Neurol. 51, 11–13 (2002).
Furlan, A. J. et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 37, 1227–1231 (2006).
Hacke, W. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 8, 141–150 (2009).
Donnan, G. A., Baron, J. C., Ma, H. & Davis, S. M. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 8, 261–269 (2009).
Siewert, B., Wielopolski, P. A., Schlaug, G., Edelman, R. R. & Warach, S. STAR MR angiography for rapid detection of vascular abnormalities in patients with acute cerebrovascular disease. Stroke 28, 1211–1215 (1997).
Petersen, E. T., Mouridsen, K. & Golay, X. The QUASAR reproducibility study, Part II: results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage 49, 104–113 (2010).
Berrington de Gonzalez, A. et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch. Intern. Med. 169, 2071–2077 (2009).
Smith-Bindman, R. et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 169, 2078–2086 (2009).
Wintermark, M. & Lev, M. H. FDA investigates the safety of brain perfusion CT. AJNR Am. J. Neuroradiol. 31, 2–3 (2010).
Martin, D. R. et al. Nephrogenic systemic fibrosis versus contrast-induced nephropathy: risks and benefits of contrast-enhanced MR and CT in renally impaired patients. J. Magn. Reson. Imaging 30, 1350–1356 (2009).
Katzberg, R. W. & Lamba, R. Contrast-induced nephropathy after intravenous administration: fact or fiction? Radiol. Clin. North Am. 47, 789–800 (2009).
Thomsen, H. S., Morcos, S. K. & Barrett, B. J. Contrast-induced nephropathy: the wheel has turned 360 degrees. Acta Radiol. 49, 646–657 (2008).
Josephson, S. A., Dillon, W. P. & Smith, W. S. Incidence of contrast nephropathy from cerebral CT angiography and CT perfusion imaging. Neurology 64, 1805–1806 (2005).
Krol, A. L. et al. Incidence of radiocontrast nephropathy in patients undergoing acute stroke computed tomography angiography. Stroke 38, 2364–2366 (2007).
Hopyan, J. J. et al. Renal safety of CT angiography and perfusion imaging in the emergency evaluation of acute stroke. AJNR Am. J. Neuroradiol. 29, 1826–1830 (2008).
Lima, F. O. et al. Functional contrast-enhanced CT for evaluation of acute ischemic stroke does not increase the risk of contrast-induced nephropathy. AJNR Am. J. Neuroradiol. (2009).
U.S. Food and Drug Administration. FDA Requests Boxed Warning for Contrast Agents Used to Improve MRI Images, [online] (2007).
Martin, D. R. Nephrogenic system fibrosis: a radiologist's practical perspective. Eur. J. Radiol. 66, 220–224 (2008).
Chrysochou, C. et al. Low risk for nephrogenic systemic fibrosis in nondialysis patients who have chronic kidney disease and are investigated with gadolinium-enhanced magnetic resonance imaging. Clin. J. Am. Soc. Nephrol. 5, 484–489 (2010).
Kanal, E., Broome, D. R., Martin, D. R. & Thomsen, H. S. Response to the FDA's May 23, 2007, nephrogenic systemic fibrosis update. Radiology 246, 11–14 (2008).
Leiner, T. & Kucharczyk, W. NSF prevention in clinical practice: summary of recommendations and guidelines in the United States, Canada, and Europe. J. Magn. Reson. Imaging 30, 1357–1363 (2009).
Thomsen, H. S. How to avoid nephrogenic systemic fibrosis: current guidelines in Europe and the United States. Radiol. Clin. North Am. 47, 871–875, vii (2009).
Acknowledgements
This work was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, NIH. Laurie Barclay, freelance writer and reviewer, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to this Review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Merino, J., Warach, S. Imaging of acute stroke. Nat Rev Neurol 6, 560–571 (2010). https://doi.org/10.1038/nrneurol.2010.129
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2010.129
This article is cited by
-
Comprehensive voxel-wise, tract-based, and network lesion mapping reveals unique architectures of right and left visuospatial neglect
Brain Structure and Function (2023)
-
Artificially-reconstructed brain images with stroke lesions from non-imaging data: modeling in categorized patients based on lesion occurrence and sparsity
Scientific Reports (2022)
-
Role of imaging in early diagnosis of acute ischemic stroke: a literature review
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2021)
-
A low-cost and shielding-free ultra-low-field brain MRI scanner
Nature Communications (2021)
-
Neurite Orientation Dispersion and Density Imaging of Rat Brain Microstructural Changes due to Middle Cerebral Artery Occlusion at a 3T MRI
Current Medical Science (2021)