Abstract
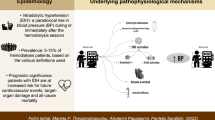
Intradialytic hypertension is not a rare complication of dialysis, with a prevalence of 5–15% among hemodialysis patients, and it seems to be associated with adverse outcomes. This complex phenomenon is not well understood, and many uncertainties exist regarding its pathophysiologic mechanisms and appropriate treatment strategies. Mechanisms that might be involved in the pathogenesis of intradialytic hypertension include extracellular volume overload, increased cardiac output, changes in electrolyte levels (particularly sodium), activation of the renin–angiotensin–aldosterone system, overactivity of the sympathetic nervous system, and endothelial cell dysfunction. Most current treatment strategies are based only on expert opinion and not on the results of randomized clinical trials, as very little data on the therapy of intradialytic hypertension are available. The most important treatment is adequate sodium and water removal, but reducing sympathetic hyperactivity and reducing endothelin-1 levels should also be considered. Well-designed, randomized clinical trials are urgently needed to better understand the pathophysiologic mechanisms of this complex phenomenon and to improve its diagnosis, prognosis and treatment.
Key Points
-
Intradialytic hypertension has a prevalence of 5–15% among patients on hemodialysis and seems to be associated with adverse outcomes
-
Mechanisms potentially involved in the onset of intradialytic hypertension include extracellular volume overload, increased cardiac output, changes in electrolyte levels, activation of the renin–angiotensin–aldosterone system and the sympathetic nervous system, and endothelial cell dysfunction
-
Very little data on the treatment of intradialytic hypertension exist; most current therapeutic strategies have been proposed from potential pathophysiologic mechanisms and are based purely on expert opinion
-
The most important treatment approach for intradialytic hypertension is adequate sodium and fluid removal; reducing sympathetic hyperactivity should also be considered
-
More information from well-conducted randomized controlled clinical trials is needed to determine pathophysiologic mechanisms and define the best treatment approach for intradialytic hypertension
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Amerling, R. C. G. et al. Complications during hemodialysis. In Clinical Dialysis (Eds Nissenson, A. R. & Gentile, D. E.) 236–267 (Appleton & Lange, Stamford, 1995).
Fellner, S. Intradialytic hypertension II. Semin. Dial. 6, 371–373 (1993).
Cirit, M. et al. 'Paradoxical' rise in blood pressure during ultrafiltration in dialysis patients. Nephrol. Dial. Transplant. 10, 1417–1420 (1995).
Mees, D. Rise in blood pressure during hemodialysis-ultrafiltration: a “paradoxical” phenomenon? Int. J. Artif. Organs 19, 569–570 (1996).
Inrig, J. K. et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 71, 454–461 (2007).
Inrig, J. K., Patel, U. D., Toto, R. D. & Szczech, L. A. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am. J. Kidney Dis. 54, 881–890 (2009).
National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations: 2006 updates: hemodialysis adequacy [online], (2006).
European Renal Association-European Dialysis and Transplant Association (ERA-EDTA). European best practice guidelines on haemodialysis. Nephrol. Dial. Transplant. 22 (Suppl. 2) (2007).
Gunal, A. I., Karaca, I., Celiker, H., Ilkay, E. & Duman, S. Paradoxical rise in blood pressure during ultrafiltration is caused by increased cardiac output. J. Nephrol. 15, 42–47 (2002).
Chou, K.-J. et al. Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int. 69, 1833–1838 (2006).
Batlle, D. C., von Riotte, A. & Lang, G. Delayed hypotensive response to dialysis in hypertensive patients with end-stage renal disease. Am. J. Nephrol. 6, 14–20 (1986).
Wilson, I., Shah, T. & Nissenson, A. R. Role of sodium and volume in the pathogenesis of hypertension in hemodialysis. Semin. Dial. 17, 260–264 (2004).
Davenport, A. Response to “The long forgotten salt factor”. Kidney Int. 74, 964–965 (2008).
Shaldon, S., Ozkahya, M., Oh, E., Basci, A. & Dorhout Mees, E. J. The long forgotten salt factor. Kidney Int. 74, 963–964 (2008).
Bianchi, G. et al. Role of the kidney in 'salt and water dependent hypertension' of end-stage disease. Clin. Sci. 42, 47–55 (1972).
Vertes, V., Cangiano, J. L., Berman, L. B. & Gould, A. Hypertension in end-stage renal disease. N. Engl. J. Med. 280, 978–981 (1969).
Bazzato, G. et al. Prevention of intra- and postdialytic hypertensive crises by captopril. Contrib. Nephrol. 41, 292–298 (1984).
Laederach, K. & Weidmann, P. Plasma and urinary catecholamines as related to renal function in man. Kidney Int. 31, 107–111 (1987).
Converse, R. L. et al. Sympathetic overactivity in patients with chronic renal failure. N. Engl. J. Med. 327, 1912–1918 (1992).
Blankestijn, P. J., Ligtenberg, G., Klein, I. H. & Koomans, H. A. Sympathetic overactivity in renal failure controlled by ACE inhibition: clinical significance. Nephrol. Dial. Transplant. 15, 755–758 (2000).
Chen, J., Gul, A. & Sarnak, M. J. Management of intradialytic hypertension: the ongoing challenge. Semin. Dial. 19, 141–145 (2006).
Cice, G. et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J. Am. Coll. Cardiol. 41, 1438–1444 (2003).
http://clinicaltrials.gov/ct2/show/NCT00827775?term=intradialytic+hypertension&rank=1 (2009).
[No authors listed] Medication use among dialysis patients in the DMMS: United States Renal Data System. Dialysis Morbidity and Mortality Study. Am. J. Kidney Dis. 32, S60–S68 (1998).
Zilch, O. et al. Sympathetic hyperactivity in haemodialysis patients is reduced by short daily hemodialysis. J. Hypertens. 25, 1285–1289 (2007).
Fagugli, R. M. et al. Effect of short daily hemodialysis and extended standard hemodialysis on blood pressure and cardiac hypertrophy: a comparative study. J. Nephrol. 19, 77–83 (2006).
Locatelli, F. et al. Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol. Dial. Transplant. 19, 785–796 (2004).
Wen, H. T. N. C. S. The Yellow Emperor's Classic of Internal Medicine 141 (University of California Press, Berkeley, 1966).
Twardowski, Z. J. Sodium, hypertension, and an explanation of the “lag phenomenon” in hemodialysis patients. Hemodial. Int. 12, 412–425 (2008).
He, F. J., Markandu, N. D. & MacGregor, G. A. Importance of the renin system for determining blood pressure fall with salt restriction in hypertensive and normotensive whites. Hypertension 38, 321–325 (2001).
MacGregor, G. A. Double-blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet 1, 351–355 (1982).
MacGregor, G. A., Markandu, N. D., Sagnella, G. A., Singer, D. & Cappuccio, F. P. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet 2, 1244–1247 (1989).
Woolfson, R. G. & de Wardener, H. E. Primary renal abnormalities in hereditary hypertension. Kidney Int. 50, 717–731 (1996).
de Wardener, H. E., He, F. J. & MacGregor, G. A. Plasma sodium and hypertension. Kidney Int. 66, 2454–2466 (2004).
Gu, J. W. et al. Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension 31, 1083–1087 (1998).
Locatelli, F., Colzani, S., D'Amico, M., Manzoni, C. & Di Filippo, S. Dry weight and sodium balance. Semin. Nephrol. 21, 291–297 (2001).
Locatelli, F., Di Filippo, S. & Manzoni, C. Relevance of the conductivity kinetic model in the control of sodium pool. Kidney Int. Suppl. 58, S89–S95 (2000).
Flanigan, M. J. Role of sodium in hemodialysis. Kidney Int. Suppl. 58, S72–S78 (2000).
Lambie, S. H., Taal, M. W., Fluck, R. J. & McIntyre, C. W. Online conductivity monitoring: validation and usefulness in a clinical trial of reduced dialysate conductivity. ASAIO J. 51, 70–76 (2005).
Levin, N. W., Zhu, F. & Keen, M. Interdialytic weight gain and dry weight. Blood Purif. 19, 217–221 (2001).
Jindal, K. et al. Chapter 2: management of blood pressure in hemodialysis patients. J. Am. Soc. Nephrol. 17, S8–S10 (2006).
Santos, S. F. F. & Peixoto, A. J. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 3, 522–530 (2008).
Gotch, F. A., Lam, M. A., Prowitt, M. & Keen, M. Preliminary clinical results with sodium-volume modeling of hemodialysis therapy. Proc. Clin. Dial. Transplant Forum 10, 12–17 (1980).
Oliver, M. J., Edwards, L. J. & Churchill, D. N. Impact of sodium and ultrafiltration profiling on hemodialysis-related symptoms. J. Am. Soc. Nephrol. 12, 151–156 (2001).
Locatelli, F. et al. Effect of on-line conductivity plasma ultrafiltrate kinetic modeling on cardiovascular stability of hemodialysis patients. Kidney Int. 53, 1052–1060 (1998).
Charra, B., Bergstrom, J. & Scribner, B. H. Blood pressure control in dialysis patients: importance of the lag phenomenon. Am. J. Kidney Dis. 32, 720–724 (1998).
Dolson, G. M., Ellis, K. J., Bernardo, M. V., Prakash, R. & Adroqué, H. J. Acute decreases in serum potassium augment blood pressure. Am. J. Kidney Dis. 26, 321–326 (1995).
Maynard, J. C., Cruz, C., Kleerekoper, M. & Levin, N. W. Blood pressure response to changes in serum ionized calcium during haemodialysis. Ann. Intern. Med. 104, 358–361 (1986).
Fellner, S. K. et al. Physiological mechanisms for calcium-induced changes in systemic arterial pressure in stable dialysis patients. Hypertension 13, 213–218 (1989).
van Kuijk, W. H. M., Mulder, A. W., Peels, C. H., Harff, G. H. & Leunissen, K. M. L. Influence of changes in ionized calcium on cardiovascular reactivity during haemodialysis. Clin. Nephrol. 47, 190–196 (1997).
van der Sande, F. M., Cheriex, E. C., van Kuijk, W. H. M. & Leunissen, K. M. L. Effect of dialysate calcium concentrations on intradialytic blood pressure course in cardiac-compromised patients. Am. J. Kidney Dis. 32, 125–131 (1998).
Gabutti, L., Bianchi, G., Soldini, D., Marone, C. & Burnier, M. Haemodynamic consequences of changing bicarbonate and calcium concentrations in haemodialysis fluids. Nephrol. Dial. Transplant. 24, 973–981 (2009).
Kyriazis, J., Stamatiadis, D. & Mamouna, A. Intradialytic and interdialytic effects of treatment with 1.25 and 1.75 mmol/l of calcium dialysate on arterial compliance in patients on hemodialysis. Am. J. Kidney Dis. 35, 1096–1103 (2000).
Kyriazis, J. et al. Arterial stiffness alterations during hemodialysis: the role of dialysate calcium. Nephron Clin. Pract. 106, c34–c42 (2007).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD). Kidney Int. 76, S1–S130 (2009).
Raj, D. S. et al. Hemodynamic changes during hemodialysis: role of nitric oxide and endothelin. Kidney Int. 61, 697–704 (2002).
El-Shafey, E. M., El-Nagar, G. F., Selim, M. F., El-Sorogy, H. A. & Sabry, A. A. Is there a role for endothelin-1 in the hemodynamic changes during hemodialysis? Clin. Exp. Nephrol. 12, 370–375 (2008).
Anderson, T. J., Elstein, E., Haber, H. & Charbonneau, F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study). J. Am. Coll. Cardiol. 35, 60–66 (2000).
Sola, S. et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation 111, 343–348 (2005).
Kaufmann, P. A. et al. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation 105, 452–456 (2002).
Vasa, M. et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 103, 2885–2890 (2001).
Lerman, A., Burnett, J. C. Jr, Higano, S. T., McKinley, L. J. & Holmes, D. R. Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation 97, 2123–2128 (1998).
Husain, S., Andrews, N. P., Mulcahy, D., Panza, J. A. & Quyyumi, A. A. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation 97, 716–720 (1998).
Chan, C. T., Li, S. H. & Verma, S. Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. Am. J. Physiol. Renal Physiol. 289, F679–F684 (2005).
Caglar, K. et al. Short-term treatment with sevelamer increases serum fetuin-A concentration and improves endothelial dysfunction in chronic kidney disease stage 4 patients. Clin. J. Am. Soc. Nephrol. 3, 61–68 (2008).
Herbrig, K. et al. Kidney transplantation substantially improves endothelial progenitor cell dysfunction in patients with end-stage renal disease. Am. J. Transplant. 6, 2922–2928 (2006).
Daugirdas, B. & Ing, H. Handbook of Dialysis (Eds Daugirdas, J. T. et al.) 471 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Raine, A. E. & Roger, S. D. Effects of erythropoietin on blood pressure. Am. J. Kidney Dis. 18 (Suppl. 1), 76–83 (1991).
Krapf, R. & Hulter, H. N. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin. J. Am. Soc. Nephrol. 4, 470–480 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Locatelli, F., Cavalli, A. & Tucci, B. The growing problem of intradialytic hypertension. Nat Rev Nephrol 6, 41–48 (2010). https://doi.org/10.1038/nrneph.2009.200
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.200
This article is cited by
-
Intradialytic hypertension: epidemiology and pathophysiology of a silent killer
Hypertension Research (2022)
-
Fluid Volume Expansion and Depletion in Hemodialysis Patients Lack Association with Clinical Parameters
Canadian Journal of Kidney Health and Disease (2015)
-
Postdialysis blood pressure rise predicts long-term outcomes in chronic hemodialysis patients: a four-year prospective observational cohort study
BMC Nephrology (2012)
-
Evolution of volume sensitivity during hemodialysis and ultrafiltration
Clinical Autonomic Research (2011)