Abstract
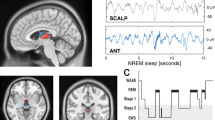
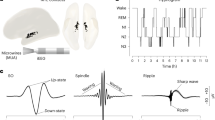
During inattentive wakefulness and non-rapid eye movement (NREM) sleep, the neocortex and thalamus cooperatively engage in rhythmic activities that are exquisitely reflected in the electroencephalogram as distinctive rhythms spanning a range of frequencies from <1 Hz slow waves to 13 Hz alpha waves. In the thalamus, these diverse activities emerge through the interaction of cell-intrinsic mechanisms and local and long-range synaptic inputs. One crucial feature, however, unifies thalamic oscillations of different frequencies: repetitive burst firing driven by voltage-dependent Ca2+ spikes. Recent evidence reveals that thalamic Ca2+ spikes are inextricably linked to global somatodendritic Ca2+ transients and are essential for several forms of thalamic plasticity. Thus, we propose herein that alongside their rhythm-regulation function, thalamic oscillations of low-vigilance states have a plasticity function that, through modifications of synaptic strength and cellular excitability in local neuronal assemblies, can shape ongoing oscillations during inattention and NREM sleep and may potentially reconfigure thalamic networks for faithful information processing during attentive wakefulness.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Niedermeyer, E. & Lopes Da Silva, F. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields (Lippincott Williams Wilkins, 2004).
Herrera, C. G. et al. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 19, 290–229 (2016).
Lorincz, M. L. & Adamantidis, A. R. Monoaminergic control of brain states and sensory processing: existing knowledge and recent insights obtained with optogenetics. Prog. Neurobiol. 151, 237–253 (2017).
Leresche, N., Lightowler, S., Soltesz, I., Jassik-Gerschenfeld, D. & Crunelli, V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J. Physiol. 441, 155–174 (1991).
Steriade, M., Nunez, A. & Amzica, F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13, 3252–3265 (1993).
Steriade, M., Contreras, D., Curró Dossi, R. & Nuñez, A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J. Neurosci. 13, 3284–3299 (1993).
Bal, T., von Krosigk, M. & McCormick, D. A. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J. Physiol. 483, 641–663 (1995).
Bal, T., von Krosigk, M. & McCormick, D. A. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J. Physiol. 483, 665–685 (1995).
Hughes, S. W., Cope, D. W., Blethyn, K. L. & Crunelli, V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron 33, 947–958 (2002).
Hughes, S. W. et al. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron 42, 253–268 (2004).
Crunelli, V., Cope, D. W. & Hughes, S. W. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium 40, 175–190 (2006).
Llinás, R. & Jahnsen, H. Electrophysiology of mammalian thalamic neurones in vitro. Nature 297, 406–408 (1982).
Deschênes, M., Paradis, M., Roy, J. P. & Steriade, M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J. Neurophysiol. 51, 1196–1219 (1984).
Attwell, D. & Gibb, A. Neuroenergetics and the kinetic design of excitatory synapses. Nat. Rev. Neurosci. 6, 841–849 (2005).
Berger, H. Uber das elektroenkephalogramm des menschen [German]. Arch. Psych. 87, 527–570 (1929).
Hughes, S. W. & Crunelli, V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 11, 357–372 (2005).
Crunelli, V., Tóth, T. I., Cope, D. W., Blethyn, K. & Hughes, S. W. The 'window' T-type calcium current in brain dynamics of different behavioural states. J. Physiol. 562, 121–129 (2005).
Lüthi, A. Sleep spindles: where they come from, what they do. Neuroscientist 20, 243–256 (2014).
Neske, G. T. The slow oscillation in cortical and thalamic networks: mechanisms and functions. Front. Neural Circ. 9, 88 (2016).
McCormick, D. A. & Bal, T. Sleep and arousal: thalamocortical mechanisms. Ann. Rev. Neurosci. 20, 185–215 (1997).
Carracedo, L. M. et al. A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms. J. Neurosci. 33, 10750–10761 (2013).
Lorincz, M. L. et al. A distinct class of slow (∼0.2-2 Hz) intrinsically bursting layer 5 pyramidal neurons determines UP/DOWN state dynamics in the neocortex. J. Neurosci. 35, 5442–5458 (2015).
Dossi, R. C., Nuñez, A. & Steriade, M. Electrophysiology of a slow (0.5-4 Hz) intrinsic oscillation of cat thalamocortical neurones in vivo. J. Physiol. 447, 215–234 (1992).
Timofeev, I. & Steriade, M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J. Neurophysiol. 76, 4152–4168 (1996).
McCormick, D. A. & Pape, H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 431, 291–318 (1990).
Bal, T. & McCormick, D. A. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J. Physiol. 468, 669–691 (1993).
Iber, C., Ancoli-Israel, S., Chesson, A. & S., Q. The new sleep scoring manual — the evidence behind the rules. J. Clin. Sleep Med. 3, 107 (2007).
Steriade, M., Nunez, A. & Amzica, F. Intracellular analysis neocortical oscillation electroencephalogram of relations between the slow (<1 Hz) and other sleep rhythms. J. Neurosci. 13, 3266–3283 (1993).
Amzica, F. & Steriade, M. Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J. Neurophysiol. 73, 20–38 (1995).
Lemieux, M., Chen, J.-Y., Lonjers, P., Bazhenov, M. & Timofeev, I. The impact of cortical deafferentation on the neocortical slow oscillation. J. Neurosci. 34, 5689–5703 (2014).
Crunelli, V., Lorincz, M. L., Errington, A. C. & Hughes, S. W. Activity of cortical and thalamic neurons during the slow (<1 Hz) rhythm in the mouse in vivo. Pflug. Arch. Eur. J. Physiol. 463, 73–88 (2012).
Sanchez-Vives, M. V. & McCormick, D. A. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 3, 1027–1034 (2000).
Zhu, L. et al. Nucleus- and species-specific properties of the slow (<1 Hz) sleep oscillation in thalamocortical neurons. Neuroscience 141, 621–636 (2006).
Blethyn, K. L., Hughes, S. W., Tóth, T. I., Cope, D. W. & Crunelli, V. Neuronal basis of the slow (<1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J. Neurosci. 26, 2474–2486 (2006).
Cunningham, M. O. et al. Neuronal metabolism governs cortical network response state. Proc. Natl Acad. Sci. USA 103, 5597–5601 (2006).
Crunelli, V. & Hughes, S. W. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat. Neurosci. 13, 9–17 (2010).
David, F. et al. Essential thalamic contribution to slow waves of natural sleep. J. Neurosci. 33, 19599–19610 (2013).
Le Bon-Jego, M. & Yuste, R. Persistently active, pacemaker-like neurons in neocortex. Front. Neurosci. 1, 123–129 (2007).
Dreyfus, F. M. et al. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of ITwindow . J. Neurosci. 30, 99–109 (2010).
Klinzing, J. G. et al. Spindle activity phase-locked to sleep slow oscillations. NeuroImage 134, 607–616 (2016).
Latchoumane, C. V., Ngo, H. V., Born, J., S. H. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435 (2017).
Morison, R. & Bassett, D. Electrical activity of the thalamus and basal ganglia in decorticate cats. J. Neurophysiol. 8, 309–314 (1945).
Steriade, M., Deschênes, M., Domich, L. & Mulle, C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J. Neurophysiol. 54, 1473–1497 (1985).
Steriade, M., Domich, L., Oakson, G. & Deschenes, M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J. Neurophysiol. 57, 260–273 (1987).
Contreras, D. & Steriade, M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J. Physiol. 490, 159–179 (1996).
Fuentealba, P. & Steriade, M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Progr. Neurobiol. 75, 125–141 (2005).
Destexhe, A., Contreras, D. & Steriade, M. Cortically-induced coherence of a thalamic-generated oscillation. Neuroscience 92, 427–443 (1999).
Contreras, D., Destexhe, A., Sejnowski, T. J. & Steriade, M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274, 771–774 (1996).
Bollimunta, A., Mo, J., Schroeder, C. E. & Ding, M. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. J. Neurosci. 31, 4935–4943 (2011).
Jensen, O., Bonnefond, M., Marshall, T. R. & Tiesinga, P. Oscillatory mechanisms of feedforward and feedback visual processing. Trends Neurosci. 38, 192–194 (2015).
Schomburg, E. W. et al. Theta phase segregation of input-specific gamma patterns in entorhinal-hippocampal networks. Neuron 84, 470–485 (2014).
Hughes, S. W. et al. Thalamic gap junctions control local neuronal synchrony and influence macroscopic oscillation amplitude during EEG alpha rhythms. Front. Psychol. 2, 193 (2011).
Lorincz, M. L., Kékesi, K. A., Juhász, G., Crunelli, V. & Hughes, S. W. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron 63, 683–696 (2009).
MacFarlane, J. G., Shahal, B., Mously, C. & Moldofsky, H. Periodic K-alpha sleep EEG activity and periodic limb movements during sleep: comparisons of clinical features and sleep parameters. Sleep 19, 200–204 (1996).
Silva, L. R., Amitai, Y. & Connorst, B. W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 251, 433–435 (1991).
Flint, A. C. & Connors, B. W. Two types of network oscillations in neocortex mediated by distinct glutamate receptor subtypes and neuronal populations. J. Neurophysiol. 75, 951–957 (1996).
Lopes Da Silva, F. H. & Storm Van Leeuwen, W. The cortical source of the alpha rhythm. Neurosci. Lett. 6, 237–241 (1977).
Lopes da Silva, F. H., Vos, J. E., Mooibroek, J. & Van Rotterdam, A. Relative contributions of intracortical and thalamo-cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroench. Clin. Neurophysiol. 50, 449–456 (1980).
Buzsáki, G. Rhythms of the Brain (Oxford Univ. Press, 2006).
Llinás, R. & Yarom, Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J. Physiol. 315, 569–584 (1981).
Stuart, G., Schiller, J. & Sakmann, B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. 505, 617–632 (1997).
Crandall, S. R., Govindaiah, G. & Cox, C. L. Low-threshold Ca2+ current amplifies distal dendritic signaling in thalamic reticular neurons. J. Neurosci. 30, 15419–15429 (2010).
Destexhe, A., Neubig, M., Ulrich, D. & Huguenard, J. Dendritic low-threshold calcium currents in thalamic relay cells. J. Neurosci. 18, 3574–3588 (1998).
Errington, A. C., Renger, J. J., Uebele, V. N. & Crunelli, V. State-dependent firing determines intrinsic dendritic Ca2+ signaling in thalamocortical neurons. J. Neurosci. 30, 14843–14853 (2010).
Kovács, K., Sik, A., Ricketts, C. & Timofeev, I. Subcellular distribution of low-voltage activated T-type Ca2+ channel subunits (Cav3.1 and Cav3.3) in reticular thalamic neurons of the cat. J. Neurosci. Res. 88, 448–460 (2010).
Williams, S. R. & Stuart, G. J. Action potential backpropagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J. Neurosci. 20, 1307–1317 (2000).
Zomorrodi, R., Kröger, H. & Timofeev, I. Modeling thalamocortical cell: impact of Ca2+ channel distribution and cell geometry on firing pattern. Front. Comp. Neurosci. 2, 5 (2008).
Connelly, W. M., Crunelli, V. & Errington, A. C. The global spike: conserved dendritic properties enable unique Ca2+ spike generation in low-threshold spiking neurons. J. Neurosci. 35, 15505–15522 (2015).
Major, G., Larkum, M. E. & Schiller, J. Active properties of neocortical pyramidal neuron dendrites. Ann. Rev. Neurosci. 36, 1–24 (2013).
Stuart, G., Spruston, N. & Häusser, M. Dendrites (Oxford Univ. Press, 2016).
Sieber, A. R., Min, R. & Nevian, T. Non-Hebbian long-term potentiation of inhibitory synapses in the thalamus. J. Neurosci. 33, 15675–15685 (2013).
Zaman, T. et al. CaV2.3 channels are critical for oscillatory burst discharges in the reticular thalamus and absence epilepsy. Neuron 70, 95–108 (2011).
Connelly, W. M., Crunelli, V. & Errington, A. C. Variable action potential backpropagation during tonic firing and low-threshold spike bursts in thalamocortical but not thalamic reticular nucleus neurons. J. Neurosci. 37, 5319–5333 (2017).
Errington, A. C., Hughes, S. W. & Crunelli, V. Rhythmic dendritic Ca2+ oscillations in thalamocortical neurons during slow non-REM sleep-related activity in vitro. J. Physiol. 590, 3691–3700 (2012).
Cueni, L. et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat. Neurosci. 11, 683–692 (2008).
Chausson, P., Leresche, N. & Lambert, R. C. Dynamics of intrinsic dendritic calcium signaling during tonic firing of thalamic reticular neurons. PLoS ONE 8, e72275 (2013).
Perez-Reyes, E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol. Rev. 83, 117–161 (2003).
Astori, S. et al. The CaV3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc. Natl Acad. Sci. USA 108, 13823–13828 (2011).
Swadlow, H. A. & Gusev, A. G. The impact of 'bursting' thalamic impulses at a neocortical synapse. Nat. Neurosci. 4, 402–408 (2001).
Reinagel, P., Godwin, D., Sherman, S. M. & Koch, C. Encoding of visual information by LGN bursts. J. Neurophysiol. 81, 2558–2569 (1999).
Sherman, S. M. & Guillery, R. W. Functional organization of thalamocortical relays. J. Neurophysiol. 76, 1367–1395 (1996).
Balduzzi, D. & Tononi, G. What can neurons do for their brain? Communicate selectivity with bursts. Theory Biosci. 132, 27–39 (2013).
Kepecs, A. & Lisman, J. Information encoding and computation with spikes and bursts. Network 14, 103–118 (2003).
Beierlein, M., Fall, C. P., Rinzel, J. & Yuste, R. Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate. J. Neurosci. 22, 9885–9894 (2002).
Rosanova, M. & Ulrich, D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J. Neurosci. 25, 9398–9405 (2005).
Hu, H. & Agmon, A. Differential excitation of distally versus proximally targeting cortical interneurons by unitary thalamocortical bursts. J. Neurosci. 36, 6906–6916 (2016).
Izhikevich, E. M., Desai, N. S., Walcott, E. C. & Hoppensteadt, F. C. Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 26, 161–167 (2003).
Guido, W. & Weyand, T. Burst responses in thalamic relay cells of the awake behaving cat. J. Neurophysiol. 74, 1782–1786 (1995).
Ortuño, T., Grieve, K. L., Cao, R., Cudeiro, J. & Rivadulla, C. Bursting thalamic responses in awake monkey contribute to visual detection and are modulated by corticofugal feedback. Front. Behav. Neurosci. 8, 198 (2014).
Luscher, C. & Malenka, R. C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 4, a005710 (2012).
Kato, H. K., Watabe, A. M. & Manabe, T. Non-Hebbian synaptic plasticity induced by repetitive postsynaptic action potentials. J. Neurosci. 29, 11153–11160 (2009).
Jones, E. G. The Thalamus (Springer, 1985).
Pigeat, R., Chausson, P., Dreyfus, F. M., Leresche, N. & Lambert, R. C. Sleep slow wave-related homo and heterosynaptic LTD of intrathalamic GABAAergic synapses: involvement of T-type Ca2+ channels and metabotropic glutamate receptors. J. Neurosci. 35, 64–73 (2015).
Astori, S. & Lüthi, A. Synaptic plasticity at intrathalamic connections via CaV3.3 T-type Ca2+ channels and GluN2B-containing NMDA receptors. J. Neurosci. 33, 624–630 (2013).
Hsu, C. L., Yang, H. W., Yen, C. T. & Min, M. Y. A requirement of low-threshold calcium spike for induction of spike-timing-dependent plasticity at corticothalamic synapses on relay neurons in the ventrobasal nucleus of rat thalamus. Chin. J. Physiol. 55, 380–389 (2012).
Hsu, C.-L., Yang, H.-W., Yen, C.-T. & Min, M.-Y. Comparison of synaptic transmission and plasticity between sensory and cortical synapses on relay neurons in the ventrobasal nucleus of the rat thalamus. J. Physiol. 588, 4347–4363 (2010).
Haas, J. S., Zavala, B. & Landisman, C. E. Activity-dependent long-term depression of electrical synapses. Science 334, 389–393 (2011).
Sevetson, J., Fittro, S., Heckman, E. & Haas, J. S. A calcium-dependent pathway underlies activity-dependent plasticity of electrical synapses in the thalamic reticular nucleus. J. Physiol. 595, 4417–4430 (2017).
Lee, S., Patrick, S. L., Richardson, K. A. & Connors, B. W. Two functionally distinct networks of gap junction-coupled inhibitory neurons in the thalamic reticular nucleus. J. Neurosci. 34, 13170–13182 (2014).
Lüthi, A. & McCormick, D. A. H-Current: properties of a neuronal and network pacemaker. Neuron 21, 9–12 (1998).
Lüthi, A. & McCormick, D. A. Modulation of a pacemaker current through Ca2+-induced stimulation of cAMP production. Nat. Neurosci. 2, 634–641 (1999).
Tononi, G. & Cirelli, C. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 62, 143–150 (2003).
Diekelmann, S. & Born, J. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010).
Watson, B. O., Levenstein, D., Greene, J. P., Gelinas, J. N. & Buzsáki, G. Network homeostasis and state dynamics of neocortical sleep. Neuron 90, 839–852 (2016).
Liu, Z.-W., Faraguna, U., Cirelli, C., Tononi, G. & Gao, X.-B. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J. Neurosci. 30, 8671–8675 (2010).
Aton, S. J. et al. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron 61, 454–466 (2009).
Tononi, G. & Cirelli, C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34 (2014).
Durkin, J. et al. Cortically coordinated NREM thalamocortical oscillations play an essential, instructive role in visual system plasticity. Proc. Natl Acad. Sci. USA 114, 10485–10490 (2017).
Acsady, L. The thalamic paradox. Nat. Neurosci. 20, 901–902 (2017).
Halassa, M. M. & Acsády, L. Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci. 39, 680–693 (2016).
Halassa, M. M. et al. State-dependent architecture of thalamic reticular subnetworks. Cell 158, 808–821 (2014).
Clemente-Perez, A. et al. Distinct thalamic reticular cell types differentially modulate normal and pathological cortical rhythms. Cell Rep. 19, 2130–2142 (2017).
Wells, M. F., Wimmer, R. D., Schmitt, L. I., Feng, G. & Halassa, M. M. Thalamic reticular impairment underlies attention deficit in Ptchd1Y/- mice. Nature 532, 58–63 (2016).
Guehl, D. et al. Tremor-related activity of neurons in the 'motor' thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur. J. Neurosci. 17, 2388–2400 (2003).
Magnin, M., Morel, A. & Jeanmonod, D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience 96, 549–564 (2000).
von Krosigk, M., Bal, T. & McCormick, D. A. Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261, 361–364 (1993).
Amzica, F. & Steriade, M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience 82, 671–686 (1998).
Contreras, D. & Steriade, M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J. Neurosci. 15, 604–622 (1995).
Acknowledgements
The authors thank C. McCafferty, R. Bódizs and P. Halász for critical comments. The authors' work is supported by the Wellcome Trust (grant 91882 to V.C.), the European Union (grant ITN-2016/722053 to V.C.), the Hungarian Scientific Research Fund (grants NF105083, NN125601 and FK123831 to M.L.L.), the Hungarian Brain Research Program (grant KTIA_NAP_13-2-2014-0014 to M.L.L.), the Centre national de la recherche scientifique (CNRS) (grant LIA528 to V.C., N.L. and R.C.L.) and the Agence Nationale de la Recherche (grants ANR-06-Neuro-050-01 and ANR-09-MNPS-035-01 to N.L. and R.C.L.). A.C.E. is a Jane Hodge Foundation Senior Research Fellow.
Author information
Authors and Affiliations
Contributions
V.C.: substantial contribution to discussion of content, writing, review, and editing of manuscript before and after submission; A.C.E.: substantial contribution to discussion of content, writing, review and editing of manuscript before submission; M.L.L.: researching data for article, substantial contribution to discussion of content, review and editing of manuscript before submission; W.M.C.: substantial contribution to discussion of content, review and editing of manuscript before submission; F.D.: substantial contribution to discussion of content, review and editing of manuscript before submission; S.W.H.: substantial contribution to discussion of content, review and editing of manuscript before submission; R.C.L.: substantial contribution to discussion of content, review and editing of manuscript before submission; N.L.: substantial contribution to discussion of content, review and editing of manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
S.W.H. is an employee of and holder of stocks in Vertex Pharmaceuticals. All other authors declare no competing financial interests.
Glossary
- Thalamocortical neurons
-
Glutamatergic thalamic neurons that project to the neocortex.
- Nucleus reticularis thalami neurons
-
GABAergic neurons of the thin, laterally located thalamic nucleus that do not project to the neocortex.
- Sleep spindles
-
Oscillatory brain activity that constitutes an electroencephalogram (EEG) hallmark of non-rapid eye movement (NREM) sleep and consists of waxing-and-waning 7–14 Hz oscillations lasting a few seconds.
- First-order
-
A functional classification of thalamic nuclei based on their main driving input: subcortical or cortical. First-order nuclei relay a particular modality of peripheral or subcortical information to a primary cortical area.
- Higher-order
-
A functional classification of thalamic nuclei that relay information from layer five cortical neurons to other cortical areas and act like a hub in cortico – thalamo – cortical information pathways.
- Intralaminar thalamic nuclei
-
A collection of thalamic nuclei involved in specific cognitive and motor functions that plays a key role in the salience of stimuli of various modalities.
- Cell-intrinsic mechanisms
-
Electrical behaviour of a neuron that results from its passive and voltage-dependent electrical properties without a contribution of the synaptic network.
- UP states
-
On the basis of their intrinsic properties and/or the influence of the synaptic network, some neurons transition between a depolarized potential, referred to as an UP state, and a hyperpolarized DOWN state.
- DOWN states
-
A state in which the neuronal membrane potential is hyperpolarized and transitions between this state and a depolarized UP state.
- T-VGCC window current
-
The small depolarizing tonic current that results from the fraction of T-type calcium channels that are open in a narrow membrane potential region around −60 mV.
- Electrotonic properties
-
The combined electrical properties of a neuron that alter the manner in which subthreshold voltage changes propagate throughout the axon and the dendritic tree.
- Backpropagating action potentials
-
(bAPs). The transient depolarization that occurs in the dendrites as a result of the generation of an action potential in the soma or axon initial segment.
- Rescale
-
Also known as synaptic rescaling; indicates the normalization of the strength of synaptic connections that had previously been either increased or decreased in response to (relatively long-term) changes in neuronal activity.
Rights and permissions
About this article
Cite this article
Crunelli, V., Lőrincz, M., Connelly, W. et al. Dual function of thalamic low-vigilance state oscillations: rhythm-regulation and plasticity. Nat Rev Neurosci 19, 107–118 (2018). https://doi.org/10.1038/nrn.2017.151
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.151
This article is cited by
-
L-Type Calcium Channel Modulates Low-Intensity Pulsed Ultrasound-Induced Excitation in Cultured Hippocampal Neurons
Neuroscience Bulletin (2024)
-
The sleeping brain’s connectivity and family environment: characterizing sleep EEG coherence in an infant cohort
Scientific Reports (2023)
-
Neocortical localization and thalamocortical modulation of neuronal hyperexcitability contribute to Fragile X Syndrome
Communications Biology (2022)
-
From choices to internal states
Nature Neuroscience (2022)
-
Thalamocortical Spectral Transmission Relies on Balanced Input Strengths
Brain Topography (2022)