Key Points
-
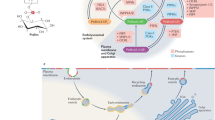
Many changes in the structure and movements of cells depend on rearrangements of the cytoskeleton. The cytoskeleton is a dynamic system of protein filaments that is regulated by dozens of proteins that are, in turn, regulated by phosphoinositides. Phosphoinositides comprise a group of anionic lipids that are generated by phosphorylation of the phospholipid phosphatidylinositol. Actin filaments are the cytoskeletal elements that are most often altered by phosphoinositide signalling.
-
Phosphoinositide-binding sites on cytoskeleton-regulating proteins are often flexible, cationic regions that allow large hinge-like transitions in the protein structure once the phosphoinositide, usually phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2), binds. The conformational change that is caused by phosphoinositides can either activate or inactivate the effects of the protein on the cytoskeleton or, in some cases, allow the protein to link the cytoskeleton to the cell membrane.
-
Manipulating phosphoinositide levels in a cell — for example, by altering the expression of enzymes that form or remove them — causes changes in the overall actin architecture of the cell. These changes are consistent with data defining how individual actin-binding-protein functions depend on phosphoinositides in vivo. Some human diseases in which cells have an abnormal cytoskeleton are associated with mutations in phosphoinositide-remodelling enzymes.
-
Soluble proteins that regulate cytoskeletal assembly do so by signalling to lipids like phosphoinositides, which are embedded in cell membranes. This allows local activation of force-producing reactions such as actin assembly to occur at the cytosol–membrane interface, where they are optimally positioned for cell motility.
-
Biochemical data and imaging of fluorescent markers for specific phosphoinositides indicate that phosphoinositides are not uniformly distributed through cell membranes but, rather, are localized in small patches or in spatial gradients. Mechanisms to account for their localization include rapid local synthesis, clustering by interaction with cationic proteins, or a combination of hydrogen-bonding and physical interactions among the lipids themselves. Direct visualization of such hypothetical domains and the determination of how they relate to cholesterol-rich lipid rafts is an area that has seen much recent activity and controversy.
Abstract
Phosphorylated derivatives of the phospholipid phosphatidylinositol, or phosphoinositides, are implicated in many aspects of cell function. Binding of phosphoinositides that are localized within cell membranes to soluble protein ligands allows spatially selective regulation at the cytoplasm–membrane interface. Recently, studies that relate phosphoinositide production to membrane domains are converging with those that show effects of these lipids on the assembly of cellular actin, and are therefore linking membrane and cytoskeletal structures in new ways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jamora, C. & Fuchs, E. Intercellular adhesion, signalling and the cytoskeleton. Nature Cell Biol. 4, E101–E108 (2002).
Sechi, A. S. & Wehland, J. Interplay between TCR signalling and actin cytoskeleton dynamics. Trends Immunol. 25, 257–265 (2004).
Forgacs, G., Yook, S. H., Janmey, P. A., Jeong, H. & Burd, C. G. Role of the cytoskeleton in signaling networks. J. Cell Sci. 117, 2769–2775 (2004).
Malm, B., Larsson, H. & Lindberg, U. The profilin–actin complex: further characterization of profilin and studies on the stability of the complex. J. Muscle Res. Cell Motil. 4, 569–588 (1983).
Burn, P., Rotman, A., Meyer, R. K. & Burger, M. M. Diacylglycerol in large α-actinin/actin complexes and in the cytoskeleton of activated platelets. Nature 314, 469–472 (1985).
Niggli, V., Dimitrov, D. P., Brunner, J. & Burger, M. M. Interaction of the cytoskeletal component vinculin with bilayer structures analyzed with a photoactivatable phospholipid. J. Biol. Chem. 261, 6912–6918 (1986).
Ito, S., Werth, D. K., Richert, N. D. & Pastan, I. Vinculin phosphorylation by the src kinase. Interaction of vinculin with phospholipid vesicles. J. Biol. Chem. 258, 14626–14631 (1983).
Anderson, R. A. & Marchesi, V. T. Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature 318, 295–298 (1985).
Lassing, I. & Lindberg, U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature 314, 472–474 (1985).
Niggli, V. Structural properties of lipid-binding sites in cytoskeletal proteins. Trends Biochem. Sci. 26, 604–611 (2001).
Yin, H. L. & Janmey, P. A. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 65, 761–789 (2003).
Hilpela, P., Vartiainen, M. K. & Lappalainen, P. Regulation of the actin cytoskeleton by PI(4,5)P2 and PI(3,4,5)P3 . Curr. Top. Microbiol. Immunol. 282, 117–163 (2004).
Popova, J. S., Greene, A. K., Wang, J. & Rasenick, M. M. Phosphatidylinositol 4,5-bisphosphate modifies tubulin participation in phospholipase Cβ1 signaling. J. Neurosci. 22, 1668–1678 (2002).
Leterrier, J. F., Kas, J., Hartwig, J., Vegners, R. & Janmey, P. A. Mechanical effects of neurofilament cross-bridges. Modulation by phosphorylation, lipids, and interactions with F-actin. J. Biol. Chem. 271, 15687–15694 (1996).
Klopfenstein, D. R., Tomishige, M., Stuurman, N. & Vale, R. D. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell 109, 347–358 (2002).
Thompson, H. M., Cao, H., Chen, J., Euteneuer, U. & McNiven, M. A. Dynamin 2 binds γ-tubulin and participates in centrosome cohesion. Nature Cell Biol. 6, 335–342 (2004).
Schafer, D. A. Regulating actin dynamics at membranes: a focus on dynamin. Traffic 5, 463–469 (2004).
Krueger, E. W., Orth, J. D., Cao, H. & McNiven, M. A. A dynamin–cortactin–Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14, 1085–1096 (2003).
Zheng, J. et al. Identification of the binding site for acidic phospholipids on the PH domain of dynamin: implications for stimulation of GTPase activity. J. Mol. Biol. 255, 14–21 (1996).
Yamamoto, M. et al. Phosphatidylinositol 4,5-bisphosphate induces actin stress-fiber formation and inhibits membrane ruffling in CV1 cells. J. Cell Biol. 152, 867–876 (2001). Showed that the stimulation of actin that was inferred from in vitro studies of protein–phosphoinositide interactions could be seen in live cells.
Rozelle, A. L. et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP–Arp2/3. Curr. Biol. 10, 311–320 (2000).
Sakisaka, T., Itoh, T., Miura, K. & Takenawa, T. Phosphatidylinositol 4,5-bisphosphate phosphatase regulates the rearrangement of actin filaments. Mol. Cell. Biol. 17, 3841–3849 (1997). Showed that some forms of actin assembly in vivo depend on the presence of cellular PtdIns(4,5)P 2.
Cunningham, C. C. et al. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J. Biol. Chem. 276, 43390–43399 (2001).
Raucher, D. et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton–plasma membrane adhesion. Cell 100, 221–228 (2000).
Miki, H., Miura, K. & Takenawa, T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 15, 5326–5335 (1996).
Shibasaki, Y. et al. Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J. Biol. Chem. 272, 7578–7581 (1997).
Kanzaki, M., Furukawa, M., Raab, W. & Pessin, J. E. Phosphatidylinositol-4,5-bisphosphate (PI4,5P2) regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J. Biol. Chem. 28 April 2004 (doi:10.1074/jbc.M401443200).
Tolias, K. F. et al. Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol. 10, 153–156 (2000).
Weernink, P. A. et al. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J. Biol. Chem. 279, 7840–7849 (2004).
Takenawa, T., Itoh, T. & Fukami, K. Regulation of phosphatidylinositol 4,5-bisphosphate levels and its roles in cytoskeletal re-organization and malignant transformation. Chem. Phys. Lipids 98, 13–22 (1999).
Niebuhr, K. et al. Conversion of PtdIns(4,5)P2 into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology. EMBO J. 21, 5069–5078 (2002).
Suchy, S. F. & Nussbaum, R. L. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am. J. Hum. Genet. 71, 1420–1427 (2002).
Funaki, M., Randhawa, P. & Janmey, P. A. Separation of insulin signaling into distinct GLUT4 translocation and activation steps. Mol. Cell. Biol. (in the press).
Venkateswarlu, K., Brandom, K. G. & Lawrence, J. L. Centaurin-α1 is an in vivo phosphatidylinositol 3,4,5-trisphosphate-dependent GTPase-activating protein for ARF6 that is involved in actin cytoskeleton organization. J. Biol. Chem. 279, 6205–6208 (2004).
Benesch, S. et al. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J. Biol. Chem. 277, 37771–37776 (2002).
Hilgemann, D. W., Feng, S. & Nasuhoglu, C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001, RE19 (2001).
Corbalan-Garcia, S., Garcia-Garcia, J., Rodriguez-Alfaro, J. A. & Gomez-Fernandez, J. C. A new phosphatidylinositol 4,5-bisphosphate-binding site located in the C2 domain of protein kinase Cα. J. Biol. Chem. 278, 4972–4980 (2003).
Frech, M. et al. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J. Biol. Chem. 272, 8474–8481 (1997).
Hresko, R. C., Murata, H. & Mueckler, M. Phosphoinositide-dependent kinase-2 is a distinct protein kinase enriched in a novel cytoskeletal fraction associated with adipocyte plasma membranes. J. Biol. Chem. 278, 21615–21622 (2003).
Fievet, B. T. et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 164, 653–659 (2004).
Kwik, J. et al. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl Acad. Sci. USA 100, 13964–13969 (2003). Distribution of phosphoinositides in the cell membrane is shown to be altered by cholesterol depletion, and an associated change in mobility of actin is linked to these membrane changes.
Cockcroft, S. (ed.) Biology of phosphoinositides (Oxford Univ. Press, Oxford, UK, 2000).
Ma, L., Cantley, L. C., Janmey, P. A. & Kirschner, M. W. Corequirement of specific phosphoinositides and small GTP binding protein cdc42 in inducing actin assembly in Xenopus egg extracts. J. Cell Biol. 140, 1125–1136 (1998).
Arioka, M., Nakashima, S., Shibasaki, Y. & Kitamoto, K. Dibasic amino acid residues at the carboxy-terminal end of kinase homology domain participate in the plasma membrane localization and function of phosphatidylinositol 5-kinase γ. Biochem. Biophys. Res. Commun. 319, 456–463 (2004).
Rohatgi, R., Ho, H. Y. & Kirschner, M. W. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 150, 1299–1310 (2000).
Rohatgi, R., Nollau, P., Ho, H. Y., Kirschner, M. W. & Mayer, B. J. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP–Arp2/3 pathway. J. Biol. Chem. 276, 26448–26452 (2001).
Sechi, A. S. & Wehland, J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P2 influences cytoskeletal protein activity at the plasma membrane. J. Cell Sci. 113, 3685–3695 (2000).
Ward, M. E., Wu, J. Y. & Rao, Y. Visualization of spatially and temporally regulated N-WASP activity during cytoskeletal reorganization in living cells. Proc. Natl Acad. Sci. USA 101, 970–974 (2004).
Mogilner, A. & Oster, G. Polymer motors: pushing out the front and pulling up the back. Curr. Biol. 13, R721–R733 (2003).
Oikawa, T. et al. PtdIns(3,4,5)P3 binding is necessary for WAVE2-induced formation of lamellipodia. Nature Cell Biol. 6, 420–426 (2004).
Merlot, S. & Firtel, R. A. Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 116, 3471–3478 (2003).
Insall, R. H. & Weiner, O. D. PIP3, PIP2, and cell movement — similar messages, different meanings? Dev. Cell 1, 743–747 (2001).
Kanaho, Y. & Suzuki, T. Phosphoinositide kinases as enzymes that produce versatile signaling lipids, phosphoinositides. J. Biochem. (Tokyo) 131, 503–509 (2002).
Doughman, R. L., Firestone, A. J., Wojtasiak, M. L., Bunce, M. W. & Anderson, R. A. Membrane ruffling requires coordination between type Iα phosphatidylinositol phosphate kinase and Rac signaling. J. Biol. Chem. 278, 23036–23045 (2003).
Doughman, R. L., Firestone, A. J. & Anderson, R. A. Phosphatidylinositol phosphate kinases put PI4,5P2 in its place. J. Membr. Biol. 194, 77–89 (2003).
Hernandez-Deviez, D. J., Roth, M. G., Casanova, J. E. & Wilson, J. M. ARNO and ARF6 regulate axonal elongation and branching through downstream activation of phosphatidylinositol 4-phosphate 5-kinase α. Mol. Biol. Cell 15, 111–120 (2004).
Ling, K., Doughman, R. L., Firestone, A. J., Bunce, M. W. & Anderson, R. A. Type I γ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420, 89–93 (2002).
Coppolino, M. G. et al. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Iα impairs localized actin remodeling and suppresses phagocytosis. J. Biol. Chem. 277, 43849–43857 (2002).
Botelho, R. J., Scott, C. C. & Grinstein, S. Phosphoinositide involvement in phagocytosis and phagosome maturation. Curr. Top. Microbiol. Immunol. 282, 1–30 (2004).
Lemmon, M. A. Phosphoinositide recognition domains. Traffic 4, 201–213 (2003).
Overduin, M., Cheever, M. L. & Kutateladze, T. G. Signaling with phosphoinositides: better than binary. Mol. Intervent. 1, 150–159 (2001).
Berg, J. S., Derfler, B. H., Pennisi, C. M., Corey, D. P. & Cheney, R. E. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J. Cell Sci. 113, 3439–3451 (2000).
Zhang, P., Talluri, S., Deng, H., Branton, D. & Wagner, G. Solution structure of the pleckstrin homology domain of Drosophila β-spectrin. Structure 3, 1185–1195 (1995).
Hyvonen, M. et al. Structure of the binding site for inositol phosphates in a PH domain. EMBO J. 14, 4676–4685 (1995).
Lemmon, M. A., Ferguson, K. M. & Abrams, C. S. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 513, 71–76 (2002).
Barret, C., Roy, C., Montcourrier, P., Mangeat, P. & Niggli, V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2-terminal domain of ezrin correlates with its altered cellular distribution. J. Cell Biol. 151, 1067–1080 (2000).
Bompard, G., Martin, M., Roy, C., Vignon, F. & Freiss, G. Membrane targeting of protein tyrosine phosphatase PTPL1 through its FERM domain via binding to phosphatidylinositol 4,5-biphosphate. J. Cell Sci. 116, 2519–2530 (2003).
Young, P. & Gautel, M. The interaction of titin and α-actinin is controlled by a phospholipid-regulated intramolecular pseudoligand mechanism. EMBO J. 19, 6331–6340 (2000).
Fukami, K. et al. Requirement of phosphatidylinositol 4,5-bisphosphate for α-actinin function. Nature 359, 150–152 (1992).
Fukami, K., Sawada, N., Endo, T. & Takenawa, T. Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle α-actinin. J. Biol. Chem. 271, 2646–2650 (1996).
Fraley, T. S. et al. Phosphoinositide binding inhibits α-actinin bundling activity. J. Biol. Chem. 278, 24039–24045 (2003).
Corgan, A. M., Singleton, C., Santoso, C. B. & Greenwood, J. A. Phosphoinositides differentially regulate α-actinin flexibility and function. Biochem. J. 378, 1067–1072 (2004).
Gilmore, A. P. & Burridge, K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature 381, 531–535 (1996).
Steimle, P. A., Hoffert, J. D., Adey, N. B. & Craig, S. W. Polyphosphoinositides inhibit the interaction of vinculin with actin filaments. J. Biol. Chem. 274, 18414–18420 (1999).
Nayal, A., Webb, D. J. & Horwitz, A. F. Talin: an emerging focal point of adhesion dynamics. Curr. Opin. Cell Biol. 16, 94–98 (2004).
Raghunathan, V., Mowery, P., Rozycki, M., Lindberg, U. & Schutt, C. Structural changes in profilin accompany its binding to phosphatidylinositol, 4,5-bisphosphate. FEBS Lett. 297, 46–50 (1992).
Xian, W. & Janmey, P. A. Dissecting the gelsolin-polyphosphoinositide interaction and engineering of a polyphosphoinositide-sensitive gelsolin C-terminal half protein. J. Mol. Biol. 322, 755–771 (2002).
Kumar, N., Zhao, P., Tomar, A., Galea, C. A. & Khurana, S. Association of villin with phosphatidylinositol 4,5-bisphosphate regulates the actin cytoskeleton. J. Biol. Chem. 279, 3096–3110 (2004). The most detailed mutational analysis so far of PtdIns(4,5)P 2 -binding domains in an actin-binding protein.
Xian, W., Vegners, R., Janmey, P. A. & Braunlin, W. H. Spectroscopic studies of a phosphoinositide-binding peptide from gelsolin: behavior in solutions of mixed solvent and anionic micelles. Biophys. J. 69, 2695–2702 (1995).
Puius, Y. A., Fedorov, E. V., Eichinger, L., Schleicher, M. & Almo, S. C. Mapping the functional surface of domain 2 in the gelsolin superfamily. Biochemistry 39, 5322–5331 (2000).
Baron, C. B., Pring, M. & Coburn, R. F. Inositol lipid turnover and compartmentation in Canine trachealis smooth muscle. Am. J. Physiol. 256, C375–C383 (1989).
Gascard, P., Sauvage, M., Sulpice, J. C. & Giraud, F. Characterization of structural and functional phosphoinositide domains in human erythrocyte membranes. Biochemistry 32, 5941–5948 (1993).
Vickers, J. D. & Mustard, J. F. The phosphoinositides exist in multiple metabolic pools in rabbit platelets. Biochem. J. 238, 411–417 (1986).
Haugh, J. M., Codazzi, F., Teruel, M. & Meyer, T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J. Cell Biol. 151, 1269–1280 (2000).
Hope, H. R. & Pike, L. J. Phosphoinositides and phosphoinositide-utilizing enzymes in detergent-insoluble lipid domains. Mol. Biol. Cell 7, 843–851 (1996).
Hannigan, M. et al. Neutrophils lacking phosphoinositide 3-kinase γ show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc. Natl Acad. Sci. USA 99, 3603–3608 (2002).
Tall, E. G., Spector, I., Pentyala, S. N., Bitter, I. & Rebecchi, M. J. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr. Biol. 10, 743–746 (2000).
Devreotes, P. & Janetopoulos, C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445–20448 (2003).
van Rheenen, J. & Jalink, K. Agonist–induced PIP2 hydrolysis inhibits cortical actin dynamics: regulation at a global but not at a micrometer scale. Mol. Biol. Cell 13, 3257–3267 (2002).
Sugiura, Y. Structure of molecular aggregates of 1-(3-sn-phosphatidyl)-L-myo-inositol 3,4-bis(phosphate) in water. Biochim. Biophys. Acta 641, 148–159 (1981).
Flanagan, L. A. et al. The structure of divalent cation-induced aggregates of PIP2 and their alteration by gelsolin and tau. Biophys. J. 73, 1440–1447 (1997).
Bettache, N. et al. Mechanical constraint imposed on plasma membrane through transverse phospholipid imbalance induces reversible actin polymerization via phosphoinositide 3-kinase activation. J. Cell Sci. 116, 2277–2284 (2003).
Cebers, A. & Janmey, P. Shape instabilities in charged lipid domains. J. Phys. Chem. B 106, 12351–12353 (2002).
McLaughlin, S., Wang, J., Gambhir, A. & Murray, D. PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 (2002).
Wang, J. et al. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J. Biol. Chem. 277, 34401–34412 (2002).
Zhang, W., Crocker, E., McLaughlin, S. & Smith, S. O. Binding of peptides with basic and aromatic residues to bilayer membranes: phenylalanine in the myristoylated alanine-rich C kinase substrate effector domain penetrates into the hydrophobic core of the bilayer. J. Biol. Chem. 278, 21459–21466 (2003).
Gambhir, A. et al. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys. J. 86, 2188–2207 (2004).
Foster, W. J. & Janmey, P. A. The distribution of polyphosphoinositides in lipid films. Biophys. Chem. 91, 211–218 (2001)
Liepina, I., Czaplewski, C., Janmey, P. & Liwo, A. Molecular dynamics study of a gelsolin-derived peptide binding to a lipid bilayer containing phosphatidylinositol 4,5-bisphosphate. Biopolymers 71, 49–70 (2003).
Redfern, D. A. & Gericke, A. Domain formation in phosphatidylinositol monophosphate/phosphatidylcholine mixed vesicles. Biophys. J. 86, 2980–2992 (2004). Identification of phosphoinositide-enriched clusters in fluid bilayer vesicles implies a new model for membrane-domain formation.
Burtnick, L. D. et al. The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell 90, 661–670 (1997).
Acknowledgements
U.L. would like to thank The Swedish National Science Research Council and The Swedish Cancer Society for long-standing financial support. P.A.J. would like to thank the US National Institute of Arthritis and Musculoskeletal and Skin Disease and the Fogarty International Center for financial support of work in this field.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Interpro
OMIM
Swiss-Prot
Glossary
- PLECKSTRIN-HOMOLOGY (PH) DOMAIN
-
A sequence of 100 amino acids that is present in many signalling molecules and binds to phosphoinositides. Pleckstrin is a protein of unknown function that was originally identified in platelets. It is a principal substrate of protein kinase C.
- RHO-FAMILY GTPases
-
Ras-related GTPases that are involved in controlling the polymerization of actin.
- LIPID RAFTS
-
Lateral aggregates of cholesterol and sphingomyelin that are thought to occur in the plasma membrane.
- RUFFLES
-
Processes that are formed by the movement of lamellipodia that are in the dynamic process of folding back onto the cell body from which they previously extended.
- GROWTH CONE
-
Motile tip of the axon or dendrite of a growing nerve cell, which spreads out into a large cone-shaped appendage.
- FOCAL-ADHESION COMPLEX
-
Focal adhesions are cellular structures that link the extracellular matrix on the outside of the cell, through integrin receptors, to the actin cytoskeleton inside the cell.
- PHAGOCYTOSIS
-
An actin-dependent process by which cells engulf external particulate material by extension and fusion of pseudopods.
- FRET
-
(fluorescence resonance energy transfer). The fluorescence energy that is transferred from one fluor excites a neighbouring fluor that then re-emits the energy at a third wavelength. Transfer occurs only if the two fluors are close, so FRET can be used to monitor real-time protein–protein interactions in living cells.
- FILOPODIA
-
Thin cellular processes containing long, unbranched, parallel bundles of actin filaments.
- PX DOMAIN
-
(phox-homology domain). A lipid- and protein-interaction domain that consists of 100–130 amino acids and is defined by sequences that are found in two components of the phagocyte NADPH oxidase (phox) complex.
- EPSIN AMINO (N)-TERMINAL HOMOLOGY DOMAIN
-
(ENTH). A phospholipid-binding motif with high affinity for PtdIns(4,5)P2.
- NUCLEAR MAGNETIC RESONANCE
-
(NMR). A technique used to determine the content, purity and molecular structure of a sample. This method is based on the fact that some atomic nuclei have a magnetic moment. When these nuclei are placed in a magnetic field and are simultaneously exposed to electromagnetic radiation, they change their energy state and absorb energy.
- CIRCULAR DICHROISM
-
An optical method measuring differential effects on light of different polarization directions to quantify the amount of α-helical and β-stranded structures within proteins.
- Z-BAND
-
A region of muscle sarcomere to which the plus ends of actin filaments are attached. It appears as a dark transverse line in micrographs.
- SARCOMERE
-
The structure within a muscle cell where actin and myosin filaments overlap to produce the movements that are required for muscle contraction. Proteins such as α-actinin were first described in these structures as proteins required to bind actin filaments in parallel arrays. Similar biochemical interactions between actin and actin-binding proteins also occur in non-muscle cells.
- β-STRAND
-
An element of protein secondary structure. Hydrogen bonds between the backbones of the same or different polypeptides stabilize arrays of parallel chains that can form larger elements resembling sheets.
- α-HELIX
-
An element of protein secondary structure in which hydrogen bonds along the backbone of a single polypeptide cause the chain to form a right-handed helix.
- FRAP
-
(fluorescence recovery after photobleaching). A live-cell imaging technique used to study the mobility of fluorescent molecules. A pulse of high intensity light is used to irreversibly photobleach a population of fluorophores in a target region. Recovery of fluorescence in the bleached region represents movement of fluorophores into that region.
- OUTER PLASMA-MEMBRANE LEAFLET
-
A lipid layer that faces the outside of the cell.
Rights and permissions
About this article
Cite this article
Janmey, P., Lindberg, U. Cytoskeletal regulation: rich in lipids. Nat Rev Mol Cell Biol 5, 658–666 (2004). https://doi.org/10.1038/nrm1434
Issue Date:
DOI: https://doi.org/10.1038/nrm1434
This article is cited by
-
Molecular mechanisms of Shigella effector proteins: a common pathogen among diarrheic pediatric population
Molecular and Cellular Pediatrics (2022)
-
Reciprocal regulation of cellular mechanics and metabolism
Nature Metabolism (2021)
-
Profilin: many facets of a small protein
Biophysical Reviews (2020)
-
Cofilin and profilin: partners in cancer aggressiveness
Biophysical Reviews (2018)
-
Effects of NMDAR Antagonist on the Regulation of P-MARCKS Protein to Aβ1−42 Oligomers Induced Neurotoxicity
Neurochemical Research (2018)