Key Points
-
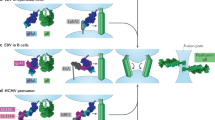
The attachment of herpesviruses to host cells requires many steps, including initial non-specific attachment, specific attachment to entry receptors and viral fusion with the cell membrane.
-
Herpes simplex virus (HSV) enters cells using the gD-binding receptors herpesvirus entry mediator, nectin-1, nectin-2 and a form of heparan sulphate, as well as the gB-binding receptor paired immunoglobulin-like type 2 receptor-α. HSV can use signalling that is induced by these receptors to regulate host immune responses.
-
The human herpesviruses express several homologues of tumour-necrosis factor (TNF) receptors, which they use to provide survival and, in some cases, transforming signals to the host cells.
-
An important mechanism of viral clearance that is used by the host immune system involves the use of the death-domain-containing TNF receptors CD95 and TNF-related apoptosis-inducing ligand receptor (TRAILR) to induce apoptosis of infected cells.
-
Adaptive immune responses are boosted through co-stimulatory signals from TNF receptors such as OX40, which increase the numbers of virus-specific T cells to limit viral replication.
-
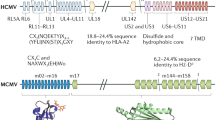
The chronic pathogen cytomegalovirus induces interferon production and lymphotoxin-β receptor activation in infected cells, which results in reduced virus production and the establishment of host–pathogen 'détente'; this allows survival of both the virus and the host cell.
Abstract
Herpesviruses have evolved numerous strategies to subvert host immune responses so they can coexist with their host species. These viruses 'co-opt' host genes for entry into host cells and then express immunomodulatory genes, including mimics of members of the tumour-necrosis factor (TNF) superfamily, that initiate and alter host-cell signalling pathways. TNF superfamily members have crucial roles in controlling herpesvirus infection by mediating the direct killing of infected cells and by enhancing immune responses. Despite these strong immune responses, herpesviruses persist in a latent form, which suggests a dynamic relationship between the host immune system and the virus that results in a balance between host survival and viral control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McGeoch, D. J., Rixon, F. J. & Davison, A. J. Topics in herpesvirus genomics and evolution. Virus Res. 117, 90–104 (2006).
French, A. R. et al. Escape of mutant double-stranded DNA virus from innate immune control. Immunity 20, 747–756 (2004).
Glenney, G. W. & Wiens, G. D. Early diversification of the TNF superfamily in teleosts: genomic characterization and expression analysis. J. Immunol. 178, 7955–7973 (2007).
Bridgham, J. T., Wilder, J. A., Hollocher, H. & Johnson, A. L. All in the family: evolutionary and functional relationships among death receptors. Cell. Death Differ. 10, 19–25 (2003).
Ware, C. F. Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 23, 787–819 (2005).
Rahman, M. M. & McFadden, G. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2, e4 (2006).
Lichtenstein, D. L., Toth, K., Doronin, K., Tollefson, A. E. & Wold, W. S. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 23, 75–111 (2004).
Benedict, C. A., Banks, T. A. & Ware, C. F. Death and survival: viral regulation of TNF signaling pathways. Curr. Opin. Immunol. 15, 59–65 (2003).
Upton, C., Macen, J., Schreiber, M. & McFadden, G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology 184, 370–382 (1991).
Spear, P. G. & Longnecker, R. Herpesvirus entry: an update. J. Virol. 77, 10179–10185 (2003).
Wang, X., Kenyon, W. J., Li, Q., Mullberg, J. & Hutt-Fletcher, L. M. Epstein–Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72, 5552–5558 (1998).
Wang, D. & Shenk, T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl Acad. Sci USA 102, 18153–18158 (2005).
Adamiak, B., Ekblad, M., Bergstrom, T., Ferro, V. & Trybala, E. Herpes simplex virus type 2 glycoprotein G is targeted by the sulfated oligo- and polysaccharide inhibitors of virus attachment to cells. J. Virol. 81, 13424–13434 (2007).
Shukla, D. & Spear, P. G. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Invest. 108, 503–510 (2001).
Spear, P. G. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6, 401–410 (2004).
Satoh, T. et al. PILRα is a herpes simplex virus-1 entry co-receptor that associates with glycoprotein B. Cell 132, 935–944 (2008). This recent paper indicates that PILRα is a new entry factor for HSV-1 and shows that the viral glycoprotein gB can also mediate specific viral attachment to host cells.
Krummenacher, C. et al. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24, 4144–4153 (2005).
Cocchi, F., Menotti, L., Mirandola, P., Lopez, M. & Campadelli-Fiume, G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72, 9992–10002 (1998).
Montgomery, R. I., Warner, M. S., Lum, B. J. & Spear, P. G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87, 427–436 (1996). This report identifies HVEM as the entry receptor for HSV-1.
Simpson, S. A. et al. Nectin-1/HveC mediates herpes simplex virus type 1 entry into primary human sensory neurons and fibroblasts. J. Neurovirol. 11, 208–218 (2005).
Manoj, S., Jogger, C. R., Myscofski, D., Yoon, M. & Spear, P. G. Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism. Proc. Natl Acad. Sci. USA 101, 12414–12421 (2004).
Taylor, J. M. et al. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 2, 19–28 (2007). This recent report is the first to show the specific contributions of each HSV-2 entry receptor in vivo , indicating that although either HVEM or nectin-1 can mediate infection, nectin-1 is mainly responsible for the formation of external lesions and neuronal infection.
Kinkade, A. & Ware, C. F. The DARC conspiracy — virus invasion tactics. Trends Immunol. 27, 362–367 (2006).
Subramanian, R. P., Dunn, J. E. & Geraghty, R. J. The nectin-1α transmembrane domain, but not the cytoplasmic tail, influences cell fusion induced by HSV-1 glycoproteins. Virology 339, 176–191 (2005).
Whitbeck, J. C. et al. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J. Virol. 71, 6083–6093 (1997).
Mauri, D. N. et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 8, 21–30 (1998).
Sedy, J. R. et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nature Immunol. 6, 90–98 (2005).
Cai, G. et al. CD160 inhibits activation of human CD4+T cells through interaction with herpesvirus entry mediator. Nature Immunol. 9, 176–185 (2008).
Schneider, K., Potter, K. G. & Ware, C. F. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol. Rev. 202, 49–66 (2004).
Murphy, K. M., Nelson, C. A. & Sedy, J. R. Balancing co-stimulation and inhibition with BTLA and HVEM. Nature Rev. Immunol. 6, 671–681 (2006).
Locksley, R. M., Killeen, N. & Lenardo, M. J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Bodmer, J. L., Schneider, P. & Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 (2002).
Compaan, D. M. et al. Attenuating lymphocyte activity: the crystal structure of the BTLA–HVEM complex. J. Biol. Chem. 280, 39553–39561 (2005).
Gonzalez, L. C. et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc. Natl Acad. Sci. USA 102, 1116–1121 (2005). This study, together with reference 27, identifies BTLA as the natural ligand for HVEM and shows that HVEM ligation of T-cell-expressed BTLA results in inhibition of T-cell activation.
Cheung, T. C. et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc. Natl Acad. Sci. USA 102, 13218–13223 (2005). In this study, the authors show that the CMV homologue of HVEM UL144 selectively interacts with the HVEM ligand BTLA and that UL144 can inhibit T-cell responses.
Carfi, A. et al. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8, 169–179 (2001).
Shiratori, I., Ogasawara, K., Saito, T., Lanier, L. L. & Arase, H. Activation of natural killer cells and dendritic cells upon recognition of a novel CD99-like ligand by paired immunoglobulin-like type 2 receptor. J. Exp. Med. 199, 525–533 (2004).
Fournier, N. et al. FDF03, a novel inhibitory receptor of the immunoglobulin superfamily, is expressed by human dendritic and myeloid cells. J. Immunol. 165, 1197–1209 (2000).
Mousseau, D. D., Banville, D., L'Abbe, D., Bouchard, P. & Shen, S. H. PILRα, a novel immunoreceptor tyrosine-based inhibitory motif-bearing protein, recruits SHP-1 upon tyrosine phosphorylation and is paired with the truncated counterpart PILRβ. J. Biol. Chem. 275, 4467–4474 (2000).
Park, S. H. et al. Rapid divergency of rodent CD99 orthologs: implications for the evolution of the pseudoautosomal region. Gene 353, 177–188 (2005).
Patel, A. et al. Herpes simplex type 1 induction of persistent NF-κB nuclear translocation increases the efficiency of virus replication. Virology 247, 212–222 (1998).
Amici, C. et al. Herpes simplex virus disrupts NF-κB regulation by blocking its recruitment on the IκBα promoter and directing the factor on viral genes. J. Biol. Chem. 281, 7110–7117 (2006).
Taddeo, B., Zhang, W., Lakeman, F. & Roizman, B. Cells lacking NF-κB or in which NF-κB is not activated vary with respect to ability to sustain herpes simplex virus 1 replication and are not susceptible to apoptosis induced by a replication-incompetent mutant virus. J. Virol. 78, 11615–11621 (2004).
Medici, M. A. et al. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor κB. J. Biol. Chem. 278, 36059–36067 (2003).
Sciortino, M. T. et al. Signaling pathway used by HSV-1 to induce NF-κB activation: possible role of herpes virus entry receptor A. Ann. N.Y. Acad. Sci. 1096, 89–96 (2007).
Cheshenko, N., Liu, W., Satlin, L. M. & Herold, B. C. Focal adhesion kinase plays a pivotal role in herpes simplex virus entry. J. Biol. Chem. 280, 31116–31125 (2005).
Cheshenko, N., Liu, W., Satlin, L. M. & Herold, B. C. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol. Biol. Cell 18, 3119–3130 (2007).
Heo, S. K. et al. HVEM signaling in monocytes is mediated by intracellular calcium mobilization. J. Immunol. 179, 6305–6310 (2007).
La, S., Kim, J., Kwon, B. S. & Kwon, B. Herpes simplex virus type 1 glycoprotein D inhibits T-cell proliferation. Mol. Cells 14, 398–403 (2002).
Sloan, D. D. et al. Inhibition of TCR signaling by herpes simplex virus. J. Immunol. 176, 1825–1833 (2006).
Koelle, D. M. et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68, 2803–2810 (1994).
Koelle, D. M. et al. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Invest. 101, 1500–1508 (1998).
Zhu, J. et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204, 595–603 (2007).
Alcami, A. Viral mimicry of cytokines, chemokines and their receptors. Nature Rev. Immunol. 3, 36–50 (2003).
Seet, B. T. et al. Poxviruses and immune evasion. Annu. Rev. Immunol. 21, 377–423 (2003).
Stanford, M. M., Werden, S. J. & McFadden, G. Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet. Res. 38, 299–318 (2007).
Coffin, W. F. 3rd, Geiger, T. R. & Martin, J. M. Transmembrane domains 1 and 2 of the latent membrane protein 1 of Epstein–Barr virus contain a lipid raft targeting signal and play a critical role in cytostasis. J. Virol. 77, 3749–3758 (2003).
Lam, N. & Sugden, B. LMP1, a viral relative of the TNF receptor family, signals principally from intracellular compartments. EMBO J. 22, 3027–3038 (2003).
Brinkmann, M. M. & Schulz, T. F. Regulation of intracellular signalling by the terminal membrane proteins of members of the gammaherpesvirinae. J. Gen. Virol. 87, 1047–1074 (2006).
Soni, V., Cahir-McFarland, E. & Kieff, E. LMP1 TRAFficking activates growth and survival pathways. Adv. Exp. Med. Biol. 597, 173–187 (2007).
Xie, P., Hostager, B. S. & Bishop, G. A. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 199, 661–671 (2004).
Xie, P. & Bishop, G. A. Roles of TNF receptor-associated factor 3 in signaling to B lymphocytes by carboxyl-terminal activating regions 1 and 2 of the EBV-encoded oncoprotein latent membrane protein 1. J. Immunol. 173, 5546–5555 (2004).
Wu, S. et al. LMP1 protein from the Epstein–Barr virus is a structural CD40 decoy in B lymphocytes for binding to TRAF3. J. Biol. Chem. 280, 33620–33626 (2005).
Shair, K. H. et al. EBV latent membrane protein 1 activates Akt, NFκB, and Stat3 in B cell lymphomas. PLoS Pathog. 3, e166 (2007).
Lambert, S. L. & Martinez, O. M. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J. Immunol. 179, 8225–8234 (2007).
Thorley-Lawson, D. A. Epstein–Barr virus: exploiting the immune system. Nature Rev. Immunol. 1, 75–82 (2001).
Schultheiss, U. et al. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 20, 5678–5691 (2001).
Luftig, M. et al. Epstein–Barr virus latent membrane protein 1 activation of NF-κB through IRAK1 and TRAF6. Proc. Natl Acad. Sci. USA 100, 15595–15600 (2003).
Atkinson, P. G., Coope, H. J., Rowe, M. & Ley, S. C. Latent membrane protein 1 of Epstein–Barr virus stimulates processing of NF-κB2 p100 to p52. J. Biol. Chem. 278, 51134–51142 (2003).
Song, Y. J., Jen, K. Y., Soni, V., Kieff, E. & Cahir-McFarland, E. IL-1 receptor-associated kinase 1 is critical for latent membrane protein 1-induced p65/RelA serine 536 phosphorylation and NF-κB activation. Proc. Natl Acad. Sci. USA 103, 2689–2694 (2006).
Luftig, M. et al. Epstein–Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK α-dependent noncanonical NF-κB activation. Proc. Natl Acad. Sci. USA 101, 141–146 (2004).
Eliopoulos, A. G. et al. TRAF1 is a critical regulator of JNK signaling by the TRAF-binding domain of the Epstein–Barr virus-encoded latent infection membrane protein 1 but not CD40. J. Virol. 77, 1316–1328 (2003).
Xie, P., Hostager, B. S., Munroe, M. E., Moore, C. R. & Bishop, G. A. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J. Immunol. 176, 5388–5400 (2006).
Wan, J. et al. Elucidation of the c-Jun N-terminal kinase pathway mediated by Estein–Barr virus-encoded latent membrane protein 1. Mol. Cell. Biol. 24, 192–199 (2004).
Uemura, N. et al. TAK1 is a component of the Epstein–Barr virus LMP1 complex and is essential for activation of JNK but not of NF-κB. J. Biol. Chem. 281, 7863–7872 (2006).
Dawson, C. W., Tramountanis, G., Eliopoulos, A. G. & Young, L. S. Epstein–Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 278, 3694–3704 (2003).
Lagunoff, M., Lukac, D. M. & Ganem, D. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 75, 5891–5898 (2001).
Lagunoff, M., Majeti, R., Weiss, A. & Ganem, D. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl Acad. Sci. USA 96, 5704–5709 (1999).
Lee, B. S. et al. Characterization of the Kaposi's sarcoma-associated herpesvirus K1 signalosome. J. Virol. 79, 12173–12184 (2005).
Tomlinson, C. C. & Damania, B. The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway. J. Virol. 78, 1918–1927 (2004).
Prakash, O. et al. Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood 105, 3987–3994 (2005).
Lee, H. et al. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol. Cell. Biol. 18, 5219–5228 (1998).
Douglas, J., Dutia, B., Rhind, S., Stewart, J. P. & Talbot, S. J. Expression in a recombinant murid herpesvirus 4 reveals the in vivo transforming potential of the K1 open reading frame of Kaposi's sarcoma-associated herpesvirus. J. Virol. 78, 8878–8884 (2004).
Brinkmann, M. M. et al. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 77, 9346–9358 (2003).
Brinkmann, M. M., Pietrek, M., Dittrich-Breiholz, O., Kracht, M. & Schulz, T. F. Modulation of host gene expression by the K15 protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 81, 42–58 (2007).
Coscoy, L. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nature Rev. Immunol. 7, 391–401 (2007).
Benedict, C. A. et al. Cutting Edge: a novel viral TNF receptor superfamily member in virulent strains of human cytomegalovirus. J. Immunol. 162, 6967–6970 (1999).
Lurain, N. S. et al. Human cytomegalovirus UL144 open reading frame: sequence hypervariability in low-passage clinical isolates. J. Virol. 73, 10040–10050 (1999).
Cha, T. A. et al. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70, 78–83 (1996).
Poole, E., King, C. A., Sinclair, J. H. & Alcami, A. The UL144 gene product of human cytomegalovirus activates NFκB via a TRAF6-dependent mechanism. EMBO J. 25, 4390–4399 (2006).
Krieg, C., Han, P., Stone, R., Goularte, O. D. & Kaye, J. Functional analysis of B and T lymphocyte attenuator engagement on CD4+ and CD8+ T cells. J. Immunol. 175, 6420–6427 (2005).
Chemnitz, J. M., Lanfranco, A. R., Braunstein, I. & Riley, J. L. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J. Immunol. 176, 6603–6614 (2006).
Nelson, C. A. et al. Structural determinants of herpesvirus entry mediator recognition by murine B and T lymphocyte attenuator. J. Immunol. 180, 940–947 (2008). This paper, together with references 17, 33 and 36, presents the known structural information about HVEM and its viral and cellular ligands gD and BTLA, showing that HVEM binds to both ligands in a similar manner and that gD undergoes a conformational change following HVEM binding.
Nakayama, T. et al. Selective induction of Th2-attracting chemokines CCL17 and CCL22 in human B cells by latent membrane protein 1 of Epstein–Barr virus. J. Virol. 78, 1665–1674 (2004).
De Trez, C. et al. The inhibitory HVEM–BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J. Immunol. 180, 238–248 (2008).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Andrews, D. M., Andoniou, C. E., Granucci, F., Ricciardi-Castagnoli, P. & Degli-Esposti, M. A. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nature Immunol. 2, 1077–1084 (2001).
Moutaftsi, M., Mehl, A. M., Borysiewicz, L. K. & Tabi, Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99, 2913–2921 (2002).
Sedger, L. M. et al. IFN-γ mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J. Immunol. 163, 920–926 (1999).
Dobbs, M. E., Strasser, J. E., Chu, C. F., Chalk, C. & Milligan, G. N. Clearance of herpes simplex virus type 2 by CD8+ T cells requires γ interferon and either perforin- or Fas-mediated cytolytic mechanisms. J. Virol. 79, 14546–14554 (2005).
Morrison, T. E., Mauser, A., Klingelhutz, A. & Kenney, S. C. Epstein–Barr virus immediate-early protein BZLF1 inhibits tumor necrosis factor α-induced signaling and apoptosis by downregulating tumor necrosis factor receptor 1. J. Virol. 78, 544–549 (2004).
Baillie, J., Sahlender, D. A. & Sinclair, J. H. Human cytomegalovirus infection inhibits tumor necrosis factor α (TNF-α) signaling by targeting the 55-kilodalton TNF-α receptor. J. Virol. 77, 7007–7016 (2003).
Browne, E. P., Wing, B., Coleman, D. & Shenk, T. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75, 12319–12330 (2001).
Le Clorennec, C. et al. EBV latency III immortalization program sensitizes B cells to induction of CD95-mediated apoptosis via LMP1: role of NF-κB, STAT1, and p53. Blood 107, 2070–2078 (2006).
Raftery, M. J. et al. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15, 997–1009 (2001).
Chiou, S. H. et al. Up-regulation of Fas ligand expression by human cytomegalovirus immediate-early gene product 2: a novel mechanism in cytomegalovirus-induced apoptosis in human retina. J. Immunol. 167, 4098–4103 (2001).
Thome, M. et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386, 517–521 (1997).
Thome, M. & Tschopp, J. Regulation of lymphocyte proliferation and death by FLIP. Nature Rev. Immunol. 1, 50–58 (2001).
Hyer, M. L., Samuel, T. & Reed, J. C. The FLIP-side of Fas signaling. Clin. Cancer Res. 12, 5929–5931 (2006).
Krammer, P. H., Arnold, R. & Lavrik, I. N. Life and death in peripheral T cells. Nature Rev. Immunol. 7, 532–542 (2007).
Snow, A. L. et al. EBV can protect latently infected B cell lymphomas from death receptor-induced apoptosis. J. Immunol. 177, 3283–3293 (2006).
Chiou, S. H. et al. The immediate early 2 protein of human cytomegalovirus (HCMV) mediates the apoptotic control in HCMV retinitis through up-regulation of the cellular FLICE-inhibitory protein expression. J. Immunol. 177, 6199–6206 (2006).
Skaletskaya, A. et al. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl Acad. Sci. USA 98, 7829–7834 (2001).
McCormick, A. L., Skaletskaya, A., Barry, P. A., Mocarski, E. S. & Goldmacher, V. S. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316, 221–233 (2003).
Mack, C., Sickmann, A., Lembo, D. & Brune, W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl Acad. Sci. USA 105, 3094–3099 (2008).
Sun, Q., Matta, H. & Chaudhary, P. M. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-κB activation. Blood 101, 1956–1961 (2003).
Guasparri, I., Wu, H. & Cesarman, E. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signalling. EMBO Rep. 7, 114–119 (2006).
Field, N. et al. KSHV vFLIP binds to IKK-γ to activate IKK. J. Cell Sci. 116, 3721–3728 (2003).
Matta, H. & Chaudhary, P. M. Activation of alternative NF-κB pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 β-converting enzyme inhibitory protein (vFLIP). Proc. Natl Acad. Sci. USA 101, 9399–9404 (2004).
Guasparri, I., Keller, S. A. & Cesarman, E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199, 993–1003 (2004).
Matta, H. et al. Induction of spindle cell morphology in human vascular endothelial cells by human herpesvirus 8-encoded viral FLICE inhibitory protein K13. Oncogene 26, 1656–1660 (2007).
Chugh, P. et al. Constitutive NF-κB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc. Natl Acad. Sci. USA 102, 12885–12890 (2005).
Wang, S. et al. K1 protein of human herpesvirus 8 suppresses lymphoma cell Fas-mediated apoptosis. Blood 109, 2174–2182 (2007).
Nanbo, A., Yoshiyama, H. & Takada, K. Epstein–Barr virus-encoded poly(A)- RNA confers resistance to apoptosis mediated through Fas by blocking the PKR pathway in human epithelial intestine 407 cells. J. Virol. 79, 12280–12285 (2005).
Reeves, M. B., Davies, A. A., McSharry, B. P., Wilkinson, G. W. & Sinclair, J. H. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 316, 1345–1348 (2007).
Kopf, M. et al. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity 11, 699–708 (1999).
Croft, M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nature Rev. Immunol. 3, 609–620 (2003).
Watts, T. H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23, 23–68 (2005).
Serghides, L. et al. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4–1BBL. J. Immunol. 175, 6368–6377 (2005).
Lee, S. W. et al. Functional dichotomy between OX40 and 4–1BB in modulating effector CD8 T cell responses. J. Immunol. 177, 4464–4472 (2006).
Lepisto, A. J., Xu, M., Yagita, H., Weinberg, A. D. & Hendricks, R. L. Expression and function of the OX40/OX40L costimulatory pair during herpes stromal keratitis. J. Leukoc. Biol. 81, 766–774 (2007).
Yu, Q. et al. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J. Immunol. 176, 2486–2495 (2006).
Humphreys, I. R. et al. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T cells: a CD4-dependent mechanism. J. Immunol. 179, 2195–2202 (2007).
Humphreys, I. R. et al. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J. Exp. Med. 204, 1217–1225 (2007). References 133 and 134 show that OX40 controls MCMV infection by co-stimulating antigen-specific T-cell responses, and that stimulating OX40 increases IFNγ production and decreases IL-10 production in the salivary glands.
Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Hislop, A. D., Taylor, G. S., Sauce, D. & Rickinson, A. B. Cellular responses to viral infection in humans: lessons from Epstein–Barr virus. Annu. Rev. Immunol. 25, 587–617 (2007).
Krug, A. et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21, 107–119 (2004).
Stetson, D. B. & Medzhitov, R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24, 93–103 (2006).
Beutler, B. et al. Genetic analysis of resistance to viral infection. Nature Rev. Immunol. 7, 753–766 (2007).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Zhang, S. Y. et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317, 1522–1527 (2007).
Benedict, C. A. et al. Lymphotoxins and cytomegalovirus cooperatively induce interferon-β, establishing host-virus detente. Immunity 15, 617–626 (2001).
Banks, T. A. et al. A lymphotoxin–IFN-β axis essential for lymphocyte survival revealed during cytomegalovirus infection. J. Immunol. 174, 7217–7225 (2005).
Schneider, K. et al. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3, 67–76 (2008). References 142 and 144 describe a mechanism by which LTβR cooperates with CMV in infected cells to induce the production of IFN, and show that B cells are the source of the initial lymphotoxin.
Iversen, A. C., Norris, P. S., Ware, C. F. & Benedict, C. A. Human NK cells inhibit cytomegalovirus replication through a noncytolytic mechanism involving lymphotoxin-dependent induction of IFN-β. J. Immunol. 175, 7568–7574 (2005).
Steed, A. L. et al. γ interferon blocks gammaherpesvirus reactivation from latency. J. Virol. 80, 192–200 (2006).
Barton, E. S., Lutzke, M. L., Rochford, R. & Virgin, H. W. 4th. α/β interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J. Virol. 79, 14149–14160 (2005).
Benedict, C. A. et al. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2, e16 (2006).
Mueller, S. N. et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317, 670–674 (2007).
Scandella, E. et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nature Immunol. 9, 667–675 (2008).
Davison, A. J., Cunningham, C., Sauerbier, W. & McKinnell, R. G. Genome sequences of two frog herpesviruses. J. Gen. Virol. 87, 3509–3514 (2006).
Aoki, T. et al. Genome sequences of three koi herpesvirus isolates representing the expanding distribution of an emerging disease threatening koi and common carp worldwide. J. Virol. 81, 5058–5065 (2007).
Bernard, D. et al. Costimulatory receptors in jawed vertebrates: conserved CD28, odd CTLA4 and multiple BTLAs. Dev. Comp. Immunol. 31, 255–271 (2007).
Zhang, B., Sun, C., Jin, S., Cascio, M. & Montelaro, R. C. Mapping of equine lentivirus receptor 1 residues critical for equine infectious anemia virus envelope binding. J. Virol. 82, 1204–1213 (2008).
Arvin, A. et al. (eds) Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. (Cambridge Univ. Press, 2007).
Geraghty, R. J., Krummenacher, C., Cohen, G. H., Eisenberg, R. J. & Spear, P. G. Entry of alpha herpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280, 1618–1620 (1998).
Shukla, D. et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99, 13–22 (1999).
Zhu, Z., Gershon, M. D., Ambron, R., Gabel, C. & Gershon, A. A. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc. Natl Acad. Sci. USA 92, 3546–3550 (1995).
Chen, J. J., Zhu, Z., Gershon, A. A. & Gershon, M. D. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell 119, 915–926 (2004).
Li, Q., Ali, M. A. & Cohen, J. I. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 127, 305–316 (2006).
Speck, P., Haan, K. M. & Longnecker, R. Epstein–Barr virus entry into cells. Virology 277, 1–5 (2000).
Wang, X., Huong, S. M., Chiu, M. L., Raab-Traub, N. & Huang, E. S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424, 456–461 (2003).
Wang, X., Huang, D. Y., Huong, S. M. & Huang, E. S. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nature Med. 11, 515–521 (2005).
Santoro, F. et al. CD46 is a cellular receptor for human herpesvirus 6. Cell 99, 817–827 (1999).
Santoro, F. et al. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 278, 25964–25969 (2003).
Mori, Y., Yang, X., Akkapaiboon, P., Okuno, T. & Yamanishi, K. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q complex associates with human CD46. J. Virol. 77, 4992–4999 (2003).
Lusso, P. et al. CD4 is a critical component of the receptor for human herpesvirus 7: interference with human immunodeficiency virus. Proc. Natl Acad. Sci. USA 91, 3872–3876 (1994).
Akula, S. M., Pramod, N. P., Wang, F. Z. & Chandran, B. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108, 407–419 (2002).
Kaleeba, J. A. & Berger, E. A. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 311, 1921–1924 (2006).
Acknowledgements
We apologize to all our colleagues whose work could not be cited because of space limitations.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Latent phase
-
A phase of a virus life cycle that is characterized by the absence of most or all viral-gene transcription despite the presence of the viral genome in host cells.
- Lytic replicative cycle
-
A phase of a virus life cycle that is characterized by active viral-gene transcription and viral-particle production and that commonly results in cell death.
- Viral glycoprotein
-
A glycoprotein that is found in the outer envelope of viruses. Of the 12 glycoproteins that are found in the outer envelope of herpesviruses, gB, gH and gL are the most highly conserved and are thought to be important for virus–host-cell membrane fusion.
- Heparan sulphate
-
A ubiquitously expressed glycosaminoglycan found on most proteoglycans. Most herpesviruses target and use heparan sulphate as a receptor for initial attachment to host cells.
- Cysteine-rich domain
-
(CRD). A protein domain that is present in several copies in most tumour-necrosis factor receptors (TNFRs) and that contains up to six cysteine residues, which form up to three disulphide bonds. CRDs are named according to their position in TNFRs and typically CRD2 and CRD3 are required for binding of the receptors to members of the TNF superfamily.
- Immunoreceptor tyrosine-based inhibitory motif
-
(ITIM). A short peptide motif (Ile/Val/Leu/Ser-X-Tyr-X-X-Leu/Val; in which X denotes any amino acid) that is present in the cytoplasmic domain of inhibitory receptors. When the tyrosine residue is phosphorylated, ITIMs recruit lipid or tyrosine phosphatases that mediate the inhibitory function of these receptors.
- Immunoreceptor tyrosine-based activation motif
-
A structural motif containing tyrosine residues, which is found in the cytoplasmic tails of several signalling molecules. The motif has the form Tyr-X-X-(Leu/Ile), in which X denotes any amino acid, and the tyrosine is a target for phosphorylation by SRC tyrosine kinases and subsequent binding of proteins containing SRC-homology 2 domains.
- Nuclear factor-κB
-
(NF-κB). A family of transcription factors (including NF-κB1 (also known as p50), NF-κB2 (also known as p52), cREL, RELA and RELB) that is important for pro-inflammatory and anti-apoptotic responses and for the development of lymphoid tissues. Each of these responses is mediated by canonical and non-canonical signalling pathways.
- Death receptor
-
A cell-surface receptor that can mediate cell death following ligand-induced trimerization. The best-studied members of the death-receptor family include tumour-necrosis factor (TNF) receptor 1, CD95 and two receptors for TNF-related apoptosis-inducing ligand (TRAILR1 and TRAILR2).
- Lipid raft
-
A cholesterol- and glycosphingolipid-rich region of the plasma membrane that provides ordered structure to the lipid bilayer and can include or exclude specific signalling molecules and complexes.
- Activator protein 1
-
A transcription factor complex composed of a heterodimer of JUN and FOS subunits that is necessary for the induction of interleukin-2 transcription in T cells. JUN and FOS subunits are members of a family of leucine-zipper-containing proteins that are induced following activation of extracellular-signal-regulated kinase and JUN N-terminal kinase.
- Mitogen-activated protein kinase
-
A family of serine/threonine kinases that are activated by various mitogenic stimuli and lead to the activation of extracellular-signal-regulated kinase, JUN N-terminal kinase or p38.
- Phosphoinositide 3-kinase
-
(PI3K). A lipid kinase that generates phosphatidylinositol-3,4,5-trisphosphate, which can be bound by pleckstrin-homology-domain-containing proteins, such as protein kinase B. PI3K activation is often associated with survival signals and the activity of anti-apoptotic proteins.
- SRC family kinases
-
A group of structurally related cytoplasmic and membrane-associated enzymes that is named after its prototypical member, SRC. In haematopoietic cells, SRC kinases are the first protein tyrosine kinases to become activated after stimulation through the immunoreceptor. They phosphorylate ITAMs of the immunoreceptors, thereby providing binding sites for SRC homology 2 (SH2)-domain-containing molecules, such as SYK.
- Fratricide
-
A form of cell killing in which one of a group of similar cells kills another member or members of the group.
Rights and permissions
About this article
Cite this article
Šedý, J., Spear, P. & Ware, C. Cross-regulation between herpesviruses and the TNF superfamily members. Nat Rev Immunol 8, 861–873 (2008). https://doi.org/10.1038/nri2434
Issue Date:
DOI: https://doi.org/10.1038/nri2434
This article is cited by
-
The kinetics of inhibitory immune checkpoints during and post-COVID-19: the knowns and unknowns
Clinical and Experimental Medicine (2023)
-
Herpes simplex virus serotype and entry receptor availability alter CNS disease in a mouse model of neonatal HSV
Pediatric Research (2014)
-
Synthesis of 3-O-sulfonated heparan sulfate octasaccharides that inhibit the herpes simplex virus type 1 host–cell interaction
Nature Chemistry (2011)
-
Natural killer cell receptor-expressing innate lymphocytes: more than just NK cells
Cellular and Molecular Life Sciences (2011)