Key Points
-
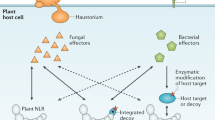
CATERPILLER proteins contain the nucleotide-binding-domain-leucine-rich-repeat (NBD-LRR) motif, and they are similar to disease resistance genes (R) found in plants that mediate immune defence to microbial pathogens.
-
MHC class II transactivator (CIITA) is a master transcriptional regulator of MHC class II genes that activates MHC class II promoters by promoting the loading of DNA-binding proteins and histone-modifying enzymes.
-
Mutations in cold induced autoinflammatory syndrome (CIAS1) are associated with a continuum of autoinflammatory diseases including familial cold autoinflammatory syndrome, Muckle–Wells syndrome, and neonatal-onset multisystem disease.
-
Cryopyrin has a key role in innate immunity by regulating cytokine responses at a transcriptional and post-translational level.
-
Interleukin-1 (IL-1) is a central mediator of CIAS1-associated disorders, as was shown by the remarkable clinical responses to IL1-targeted therapy.
-
Mediterranean fever is the locus that is mutated in familial Mediterranean fever, which is a recessively inherited systemic autoinflammatory disease with unprovoked episodes of fever, abdominal or chest pain, arthritis, or rash.
-
The amino-terminal 92 amino acids of pyrin is the prototype for the motif that bears its name, and represents the region that interacts with ASC (apoptosis-associated speck-like protein containing a CARD) to mediate IL-1β processing, nuclear-factor-κB activation, and apoptosis.
-
Both pro- and anti-inflammatory roles have been demonstrated for pyrin.
Abstract
The newly described CATERPILLER family (also known as NOD-LRR or NACHT-LRR) is comprised of proteins with a nucleotide-binding domain and a leucine-rich region. This family has gained rapid prominence because of its demonstrated and anticipated roles in immunity, cell death and growth, and diseases. CATERPILLER proteins are structurally similar to a subgroup of plant-disease-resistance (R) proteins and to the apoptotic protease activating factor 1 (APAF1). They provide positive and negative signals for the control of immune and inflammatory responses, and might represent intracellular sensors of pathogen products. Most importantly, they are genetically linked to several human immunological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yamamoto, M. & Akira, S. TIR domain-containing adaptors regulate TLR signaling pathways. Adv. Exp. Med. Biol. 560, 1–9 (2005).
Pasare, C. & Medzhitov, R. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 560, 11–18 (2005).
Beutler, B., Hoebe, K., Georgel, P., Tabeta, K. & Du, X. Genetic analysis of innate immunity: identification and function of the TIR adapter proteins. Adv. Exp. Med. Biol. 560, 29–39 (2005).
Anderson, K. V., Jurgens, G. & Nusslein-Volhard, C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42, 779–789 (1985).
Hoffman, J. A. The immune response of Drosophila. Nature 426, 33–28 (2003).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Poltorak, A., et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Harton, J. A. & Ting, J. P. Class II transactivator: mastering the art of major histocompatibility complex expression. Mol. Cell. Biol. 20, 6185–6194 (2000).
Inohara, N. & Nunez, G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene 20, 6473–6481 (2001).
Harton, J. A., Linhoff, M. W., Zhang, J. & Ting, J. P. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J. Immunol. 169, 4088–4093 (2002). References 10 and 13 report the discovery of the CATERPILLER family, also known as the NOD-LRR family.
Ting, J. P. & Davis, B. K. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu. Rev. Immunol. 23, 387–414 (2005).
Steimle, V., Otten, L. A., Zufferey, M. & Mach, B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 75, 135–146 (1993). This paper reports the discovery of CIITA and its genetic linkage to type II group A BLS.
Inohara, N. & Nunez, G. NODs: intracellular proteins involved in inflammation and apoptosis. Nature Rev. Immunol. 3, 371–382 (2003).
Inohara, Chamaillard, McDonald, C. & Nunez, G. NOD-LRR proteins: role in host–microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74, 355–383 (2005).
Martinon, F. & Tschopp, J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 26, 447–454 (2005).
Koonin, E. V. & Aravind, L. The NACHT family- a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem. Sci. 25, 223–224 (2000).
Roy, N. et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80, 167–178 (1995).
van der Steege, G., et al. A provisional transcript map of the spinal muscular atrophy (SMA) critical region. Eur. J. Hum. Genet. 3, 87–95 (1995).
Manji, G. A. et al. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-κ B. J. Biol. Chem. 277, 11570–11575 (2002). References 19, 47 and 132 116 show the association of pyrin with ASC and outcomes of this association.
Fiorentino, L., et al. A novel PAAD-containing protein that modulates NF-κB induction by cytokines tumor necrosis factor-α and interleukin-1β. J. Biol. Chem. 277, 35333–35340 (2002).
Tschopp, J., Martinon, F. & Burns, K. NALPs: a novel protein family involved in inflammation. Nature Rev. Mol. Cell Biol. 4, 95–104 (2003).
Stehlik, C. & Reed, J. C. The PYRIN connection: novel players in innate immunity and inflammation. J. Exp. Med. 200, 551–558 (2004).
Ausubel, F. M. Are innate immune signaling pathways in plants and animals conserved? Nature Immunol. 6, 973–979 (2005).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Srinivasula, S. M., Ahmad, M., Fernandes-Alnemri, T. & Alnemri, E. S. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1, 949–957 (1998).
Riedl, S. J., Li, W., Chao, Y., Schwarzenbacher, R. & Shi, Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature 434, 926–933 (2005).
Ting, J. P. & Trowsdale, J. Genetic control of MHC class II expression. Cell 109, S21–S33 (2002).
Reith, W. & Mach, B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 19, 331–373 (2001).
Swanberg, M. et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nature Genet. 37, 486–494 (2005). References 29 and 63 report the association of SNPs in the promoter of CIITA with multiple sclerosis, rheumatoid arthritis and myocardial infarction.
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nature Genet. 29, 301–305 (2001). References 30–32 and 87–88 report the association of CIAS1 mutations in autoinflammatory diseases.
Aganna, E. et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452 (2002).
Dode, C. et al. New mutations of CIAS1 that are responsible for Muckle–Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am. J. Hum. Genet. 70, 1498–1506 (2002).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Ogura, Y., et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606 (2001).
Miceli-Richard, C., et al. CARD15 mutations in Blau syndrome. Nature Genet. 29, 19–20 (2001).
Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 90, 797–807 (1997). References 36, 37 and 100 represent the data that mapped the MEFV locus.
A candidate gene for familial Mediterranean fever. The French FMF Consortium. Nature Genet 17, 25–31 (1997).
Stojanov, S. & Kastner, D. L. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr. Opin. Rheumatol. 17, 586–599 (2005).
Inohara, Chamaillard, McDonald, C. & Nunez, G. Annu. Rev. Biochem. 74, 355–383 (2005).
Girardin, S. E. & Philpott, D. J. Mini-review: the role of peptidoglycan recognition in innate immunity. Eur. J. Immunol. 34, 1777–1782 (2004).
Conti, B. J. et al. CATERPILLER 16. 2 (CLR16. 2), a novel NBD/LRR family member that negatively regulates T cell function. J. Biol. Chem. 280, 18375–18385 (2005).
Kinoshita, T., Wang, Y., Hasegawa, M., Imamura, R. & Suda, T. PYPAF3, a PYRIN-containing APAF-1-like protein, is a feedback regulator of caspase-1-dependent interleukin-1β secretion. J. Biol. Chem. 280, 21720–21725 (2005).
Bruey, J. M. et al. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-κB and caspase-1 activation in macrophages. J. Biol. Chem. 279, 51897–51907 (2004).
Williams, K. L. et al. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor α-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J. Biol. Chem. 280, 39914–39924 (2005).
Wang, L. et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 (2002).
Grenier, J. M. et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-κB and caspase-1. FEBS Lett. 530, 73–78 (2002).
Yu, J. W. et al. Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell. Death Differ. 13, 236–249 (2006).
Watanabe, T., Kitani, A., Murray, P. J. & Strober, W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nature Immunol. 5, 800–808 (2004).
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Maeda, S. et al. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1β processing. Science 307, 734–738 (2005).
Strober, M. P., Kitani A, and Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature Rev. Immunol. 6, 9–20 (2006).
Nekrep, N., Fontes, J. D., Geyer, M. & Peterlin, B. M. When the lymphocyte loses its clothes. Immunity 18, 453–457 (2003).
DeSandro, A., Nagarajan, U. M. & Boss, J. M. The bare lymphocyte syndrome: molecular clues to the transcriptional regulation of major histocompatibility complex class II genes. Am. J. Hum. Genet. 65, 279–286 (1999).
Kovats, S. et al. Coordinate defects in human histocompatibility leukocyte antigen class II expression and antigen presentation in bare lymphocyte syndrome. J. Exp. Med. 179, 2017–2022 (1994).
van Eggermond, M. C., Rijkers, G. T., Kuis, W., Zegers, B. J. & van den Elsen, P. J. T cell development in a major histocompatibility complex class II-deficient patient. Eur. J. Immunol. 23, 2585–2591 (1993).
Saleem, M. A., Arkwright, P. D., Davies, E. G., Cant, A. J. & Veys, P. A. Clinical course of patients with major histocompatibility complex class II deficiency. Arch. Dis. Child. 83, 356–359 (2000).
Martin, B. K. et al. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity 6, 591–600 (1997).
Gobin, S. J., Peijnenburg, A., Keijsers, V. & van den Elsen, P. J. Site α is crucial for two routes of IFN γ-induced MHC class I transactivation: the ISRE-mediated route and a novel pathway involving CIITA. Immunity 6, 601–611 (1997).
Cressman, D. E., Chin, K. C., Taxman, D. J. & Ting, J. P. A defect in the nuclear translocation of CIITA causes a form of type II bare lymphocyte syndrome. Immunity 10, 163–171 (1999).
Quan, V., Towey, M., Sacks, S. & Kelly, A. P. Absence of MHC class II gene expression in a patient with a single amino acid substitution in the class II transactivator protein CIITA. Immunogenetics 49, 957–963 (1999).
Wiszniewski, W., et al. Mutation in the class II trans-activator leading to a mild immunodeficiency. J. Immunol. 167, 1787–1794 (2001).
Reith, W., Leibundgut-Landmann, S. & Waldburger, J. M. Regulation of MHC class II gene expression by the class II transactivator. Nature Rev. Immunol. 5, 793–806 (2005). This paper reviews the promoters of CIITA.
Patarroyo, J. C. et al. Single nucleotide polymorphisms in MHC2TA, the gene encoding the MHC class II transactivator (CIITA). Genes Immun. 3, 34–37 (2002).
Piskurich, J. F., Wang, Y., Linhoff, M. W., White, L. C. & Ting, J. P. Identification of distinct regions of 5′ flanking DNA that mediate constitutive, IFN-γ, STAT1, and TGF-β-regulated expression of the class II transactivator gene. J. Immunol. 160, 233–240 (1998).
Zika, E. & Ting, J. P. Epigenetic control of MHC-II: interplay between CIITA and histone-modifying enzymes. Curr. Opin. Immunol. 17, 58–64 (2005).
Zhu, X. S. et al. Transcriptional scaffold: CIITA interacts with NF-Y, RFX, and CREB to cause stereospecific regulation of the class II major histocompatibility complex promoter. Mol. Cell. Biol. 20, 6051–6061 (2000).
DeSandro, A. M., Nagarajan, U. M. & Boss, J. M. Associations and interactions between bare lymphocyte syndrome factors. Mol. Cell. Biol. 20, 6587–6599 (2000).
Linhoff, M. W., Harton, J. A., Cressman, D. E., Martin, B. K. & Ting, J. P. Two distinct domains within CIITA mediate self-association: involvement of the GTP-binding and leucine-rich repeat domains. Mol. Cell. Biol. 21, 3001–3011 (2001).
Sisk, T. J., Roys, S. & Chang, C. H. Self-association of CIITA and its transactivation potential. Mol. Cell. Biol. 21, 4919–4928 (2001).
Chin, K. C., Li, G. & Ting, J. P. Activation and transdominant suppression of MHC class II and HLA-DMB promoters by a series of C-terminal class II transactivator deletion mutants. J. Immunol. 159, 2789–2794 (1997).
Hake, S. B. et al. CIITA leucine-rich repeats control nuclear localization, in vivo recruitment to the major histocompatibility complex (MHC) class II enhanceosome, and MHC class II gene transactivation. Mol. Cell. Biol. 20, 7716–7725 (2000).
Harton, J. A., O'Connor, W. Jr, Conti, B. J., Linhoff, M. W. & Ting, J. P. Leucine-rich repeats of the class II transactivator control its rate of nuclear accumulation. Hum. Immunol. 63, 588–601 (2002).
Camacho-Carvajal, M. M., Klingler, S., Schnappauf, F., Hake, S. B. & Steimle, V. Importance of class II transactivator leucine-rich repeats for dominant-negative function and nucleo-cytoplasmic transport. Int. Immunol. 16, 65–75 (2004).
Kretsovali, A., et al. Involvement of CREB binding protein in expression of major histocompatibility complex class II genes via interaction with the class II transactivator. Mol. Cell. Biol. 18, 6777–6783 (1998).
Fontes, J. D., Kanazawa, S., Jean, D. & Peterlin, B. M. Interactions between the class II transactivator and CREB binding protein increase transcription of major histocompatibility complex class II genes. Mol. Cell. Biol. 19, 941–947 (1999).
Zika, E., Fauquier, L., Vandel, L. & Ting, J. P. Interplay among coactivator-associated arginine methyltransferase 1, CBP, and CIITA in IFN-γ-inducible MHC-II gene expression. Proc. Natl Acad. Sci. USA 102, 16321–16326 (2005).
Kara, C. J. & Glimcher, L. H. In vivo footprinting of MHC class II genes: bare promoters in the bare lymphocyte syndrome. Science 252, 709–712 (1991).
Wright, K. L., et al. CIITA stimulation of transcription factor binding to major histocompatibility complex class II and associated promoters in vivo. Proc. Natl Acad. Sci. USA 95, 6267–6272 (1998).
Beresford, G. W. & Boss, J. M. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nature Immunol. 2, 652–657 (2001).
Raval, A. et al. Transcriptional coactivator, CIITA, is an acetyltransferase that bypasses a promoter requirement for TAFII250. Mol. Cell. 7, 105–115 (2001). This paper shows that CIITA alters histone acetylation.
Zika, E., Greer, S. F., Zhu, X. S. & Ting, J. P. Histone deacetylase 1/mSin3A disrupts γ interferon-induced CIITA function and major histocompatibility complex class II enhanceosome formation. Mol. Cell. Biol. 23, 3091–3102 (2003).
Wang, A. H. et al. Identification of the ankyrin repeat proteins ANKRA and RFXANK as novel partners of class IIa histone deacetylases. J. Biol. Chem. 280, 29117–29127 (2005).
Harton, J. A., Cressman, D. E., Chin, K. C., Der, C. J. & Ting, J. P. GTP binding by class II transactivator: role in nuclear import. Science 285, 1402–1405 (1999). This paper shows that CIITA is a nucleotide-binding protein.
Kretsovali, A., Spilianakis, C., Dimakopoulos, A., Makatounakis, T. & Papamatheakis, J. Self-association of class II transactivator correlates with its intracellular localization and transactivation. J. Biol. Chem. 276, 32191–32197 (2001).
Tosi, G., Jabrane-Ferrat, N. & Peterlin, B. M. Phosphorylation of CIITA directs its oligomerization, accumulation and increased activity on MHCII promoters. EMBO J. 21, 5467–5476 (2002).
Raval, A., Weissman, J. D., Howcroft, T. K. & Singer, D. S. The GTP-binding domain of class II transactivator regulates its nuclear export. J. Immunol. 170, 922–930 (2003).
Aksentijevich, I. et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 46, 3340–3348 (2002).
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002).
Hoffman, H. M. et al. Fine structure mapping of CIAS1: identification of an ancestral haplotype and a common FCAS mutation, L353P. Hum. Genet. 112, 209–216 (2003).
Hoffman, H. M., Wanderer, A. A. & Broide, D. H. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J. Allergy Clin. Immunol. 108, 615–620 (2001).
Muckle, T. J. The 'Muckle–Wells' syndrome. Br. J. Dermatol. 100, 87–92 (1979).
Prieur, A. M. et al. A chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome. A specific entity analysed in 30 patients. Scand. J. Rheumatol. Suppl. 66, 57–68 (1987).
Neven, B. et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood 103, 2809–2815 (2004).
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325 (2004). This paper shows the involvement of cryopyrin in the inflammasome, a protein scaffold involved in caspase-1 activation and subsequent interleukin-1 release.
Stack, J. H. et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J. Immunol. 175, 2630–2634 (2005).
Anderson, J. P. et al. Structural, expression, and evolutionary analysis of mouse CIAS1. Gene 338, 25–34 (2004).
Ogura, Y. et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276, 4812–4818 (2001).
Inohara, N. et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278, 5509–5512 (2003).
Dowds, T. A. et al. Regulation of cryopyrin/Pypaf1 signaling by pyrin, the familial Mediterranean fever gene product. Biochem. Biophys. Res. Commun. 302, 575–580 (2003).
Stehlik, C. et al. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J. Exp. Med. 196, 1605–1615 (2002).
O'Connor, W. Jr, Harton, J. A., Zhu, X., Linhoff, M. W. & Ting, J. P. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1. 1 is an inducible inflammatory mediator with NF-κB suppressive properties. J. Immunol. 171, 6329–6333 (2003). References 102–104 show a role for cryopyrin in IL-1 production to a range of stimuli.
Kanneganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 11 Jan 2006 (10.1038/nature04517).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 11 Jan 2006 (10.1038/nature04515).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 11 Jan 2006 (10.1038/nature04516) .
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10, 417–426 (2002).
Dowds, T. A., Masumoto, J., Zhu, L., Inohara, N. & Nunez, G. Cryopyrin-induced interleukin 1β secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J. Biol. Chem. 279, 21924–21928 (2004).
Martinon, F., Agostini, L., Meylan, E. & Tschopp, J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14, 1929–1934 (2004).
Kastner, D. L. A. in Arthritis and Allied Conditions. 15th edn, (eds Koopman, W.J and Moreland, L.W), 1411–1461 (Lippincott Williams and Wilkins, 2005).
Group, T. F. S. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore) 84, 1–11 (2005).
Imirzalioglu, N., Dursun, A., Tastan, B., Soysal, Y. & Yakicier, M. C. MEFV gene is a probable susceptibility gene for Behcet's disease. Scand. J. Rheumatol. 34, 56–58 (2005).
Cattan, D., Notarnicola, C., Molinari, N. & Touitou, I. Inflammatory bowel disease in non-Ashkenazi Jews with familial Mediterranean fever. Lancet 355, 378–379 (2000).
Fidder, H. H. et al. Crohn disease in patients with familial Mediterranean fever. Medicine (Baltimore) 81, 411–416 (2002).
Ozen, S. et al. Mutations in the gene for familial Mediterranean fever: do they predispose to inflammation? J. Rheumatol. 30, 2014–2018 (2003).
Ptacek, L. J. et al. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell 67, 1021–1027 (1991).
Weatherall, D. J., Clegg, J. B., Higgs, D. R. & Wood, W. G. in The Metabolic Bases of Inherited Disease. (eds Scriver, C.R et al.) 3417–3484 (McGraw-Hill, 1995).
Stoffman, N. et al. Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups. Eur. J. Hum. Genet. 8, 307–310 (2000).
Gershoni-Baruch, R., Shinawi, M., Leah, K., Badarnah, K. & Brik, R. Familial Mediterranean fever: prevalence, penetrance and genetic drift. Eur. J. Hum. Genet. 9, 634–637 (2001).
Kogan, A. et al. Common MEFV mutations among Jewish ethnic groups in Israel: high frequency of carrier and phenotype III states and absence of a perceptible biological advantage for the carrier state. Am. J. Med. Genet. 102, 272–276 (2001).
Yilmaz, E. et al. Mutation frequency of Familial Mediterranean Fever and evidence for a high carrier rate in the Turkish population. Eur. J. Hum. Genet. 9, 553–555 (2001).
Tunca, M. et al. Acute phase response and evolution of familial Mediterranean fever. Lancet 353, 1415 (1999).
Poland, D. C. et al. Specific glycosylation of α1-acid glycoprotein characterises patients with familial Mediterranean fever and obligatory carriers of MEFV. Ann. Rheum. Dis. 60, 777–780 (2001).
Schaner, P. et al. Episodic evolution of pyrin in primates: human mutations recapitulate ancestral amino acid states. Nature Genet. 27, 318–321 (2001).
Chae, J. J. et al. Isolation, genomic organization, and expression analysis of the mouse and rat homologs of MEFV, the gene for familial mediterranean fever. Mamm. Genome 11, 428–435 (2000).
Centola, M. et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood 95, 3223–3231 (2000).
Diaz, A. et al. Lipopolysaccharide-induced expression of multiple alternatively spliced MEFV transcripts in human synovial fibroblasts: a prominent splice isoform lacks the C-terminal domain that is highly mutated in familial Mediterranean fever. Arthritis Rheum. 50, 3679–3689 (2004).
Chen, X. et al. The familial mediterranean fever protein interacts and colocalizes with a putative Golgi transporter. Proc. Soc. Exp. Biol. Med. 224, 32–40 (2000).
Papin, S. et al. Alternative splicing at the MEFV locus involved in familial Mediterranean fever regulates translocation of the marenostrin/pyrin protein to the nucleus. Hum. Mol. Genet. 9, 3001–3009 (2000).
Tidow, N. et al. Hematopoietic-specific expression of MEFV, the gene mutated in familial Mediterranean fever, and subcellular localization of its corresponding protein, pyrin. Blood 95, 1451–1455 (2000).
Mansfield, E., et al. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood 98, 851–859 (2001).
Jeru, I. et al. Interaction of pyrin with 14. 3. 3 in an isoform-specific and phosphorylation-dependent manner regulates its translocation to the nucleus. Arthritis Rheum. 52, 1848–1857 (2005).
Bertin, J. & DiStefano, P. S. The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ 7, 1273–1274 (2000).
Richards, N. et al. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem. 276, 39320–39329 (2001).
Chae, J. J. et al. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell 11, 591–604 (2003). This paper describes the phenotype of a mouse with targeted mutation of the pyrin gene.
Masumoto, J. et al. ASC is an activating adaptor for NF-κB and caspase-8-dependent apoptosis. Biochem. Biophys. Res. Commun. 303, 69–73 (2003).
Perron, M. J. et al. TRIM5α mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl Acad. Sci USA 101, 11827–11832 (2004).
Song, B. et al. Retrovirus restriction by TRIM5α variants from Old World and New World primates. J. Virol. 79, 3930–3937 (2005).
Hawkins, P. N., Lachmann, H. J. & McDermott, M. F. Interleukin-1-receptor antagonist in the Muckle–Wells syndrome. N. Engl. J. Med. 348, 2583–2584 (2003). References 137–139 show that IL-1-targeted therapeutics effectively eliminated symptoms in CIAS1 mutation-associated disorders.
Lovell, D. J., Bowyer, S. L. & Solinger, A. M. Interleukin-1 blockade by anakinra improves clinical symptoms in patients with neonatal-onset multisystem inflammatory disease. Arthritis Rheum. 52, 1283–1286 (2005).
Hoffman, H. M. et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet 364, 1779–1785 (2004).
Acknowledgements
We would like to thank B. Davis for his helpful discussions, and acknowledge the support of National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Paralogues
-
Homologous sequences found within a single species.
- Dorsal
-
Rel/nuclear factor-κB transcription factor family member found in Drosophila melanogaster.
- Dorsal-related immunity factor
-
(DIF). A Drosophila melanogaster transcription factor that is a Rel-homologue, and is important for dorsal–ventral axis determination.
- MHC class II transactivator
-
(CIITA). The master transcriptional regulator of MHC class II genes.
- Penetrance
-
The frequency with which a genotype manifests itself in a given phenotype.
- Single nucleotide polymorphisms
-
(SNP). Common DNA sequence polymorphisms among individuals.
- Complementation group
-
Two genetic mutations that, when present in the same cell or organism, cause the appearance of wild-type traits.
- Quantitative trait locus
-
A region of DNA associated with a specific trait that varies in a quantitative fashion, rather than dichotomously.
- Histone-modifying enzymes
-
Enzymes such as acetylases and methylases that cause the covalent modification of histone tails.
- Positional cloning
-
The process of identifying a gene based on its chromosomal position, including genetic and physical mapping.
- Expressed sequence tags
-
A short partial cDNA sequence that represents a part of an expressed gene and can be mapped to a chromosomal position.
- Founder effect
-
High frequency of a particular allele in a population because the population is derived from a small number of founders, one or more of whom carried that allele.
- B30.2 domain
-
A 170 amino-acid globular domain found at the C-terminus of several proteins, first identified as the product of an isolated exon of the same name encoded in the MHC.
- Intragenic convergence of haplotypes
-
The identification of a shared set of DNA markers within a gene among unrelated individuals with a specific disease, suggesting a common ancestral origin of the disease-associated chromosomes.
- Behçet's disease
-
A disorder common in the Middle and Far East, characterized by oral and genital ulcerations, ocular inflammation, skin lesions, and a number of other inflammatory manifestations.
- dN/dS ratio
-
The ratio of nonsynonymous to synonymous substitutions, which varies directly with the probability of evolutionary selection at a given locus.
- 14.3.3 protein family
-
A family of proteins involved in the regulation of intracellular signaling, cell-cycle control, and apoptosis.
- Hypomorphic
-
A protein variant with reduced function.
- TRIM5α
-
A tripartite motif (TRIM) cytoplasmic protein with a carboxy-terminal B30.2 domain that was recently shown to mediate innate intracellular resistance to retroviruses.
Rights and permissions
About this article
Cite this article
Ting, JY., Kastner, D. & Hoffman, H. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol 6, 183–195 (2006). https://doi.org/10.1038/nri1788
Issue Date:
DOI: https://doi.org/10.1038/nri1788
This article is cited by
-
NLR members in inflammation-associated carcinogenesis
Cellular & Molecular Immunology (2017)
-
TRIM29 promotes DNA virus infections by inhibiting innate immune response
Nature Communications (2017)
-
Identification of a role for TRIM29 in the control of innate immunity in the respiratory tract
Nature Immunology (2016)
-
Metagenomic cross-talk: the regulatory interplay between immunogenomics and the microbiome
Genome Medicine (2015)
-
Endocrine autoimmune diseases and female infertility
Nature Reviews Endocrinology (2014)