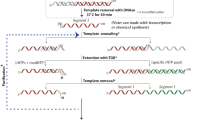
Abstract
The non-protein-coding transcriptional output of the cell is far greater than previously thought. Although the functions, if any, of the vast majority of these RNA transcripts remain elusive, out of those for which functions have already been established, most act as RNA guides for protein enzymes. Common features of these RNAs provide clues about the evolutionary constraints that led to the development of RNA-guided proteins and the specific biological environments in which target specificity and diversity are most crucial to the cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Halic, M. & Beckmann, R. The signal recognition particle and its interactions during protein targeting. Curr. Opin. Struct. Biol. 15, 116–125 (2005).
Halic, M. et al. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature 427, 808–814 (2004).
Yang, Z., Zhu, Q., Luo, K. & Zhou, Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414, 317–322 (2001).
Wassarman, K. M. & Storz, G. 6S RNA regulates E. coli RNA polymerase activity. Cell 101, 613–623 (2000).
Dubey, A. K., Baker, C. S., Romeo, T. & Babitzke, P. RNA sequence and secondary structure participate in high-affinity CsrA–RNA interaction. RNA 11, 1579–1587 (2005).
Pauler, F. M., Stricker, S. H., Warczok, K. E. & Barlow, D. P. Long-range DNase I hypersensitivity mapping reveals the imprinted Igf2r and Air promoters share cis-regulatory elements. Genome Res. 15, 1379–1387 (2005).
Blackburn, E. H. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579, 859–862 (2005).
Meister, G. & Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349 (2004).
Filipowicz, W. RNAi: the nuts and bolts of the RISC machine. Cell 122, 17–20 (2005).
Tomari, Y. & Zamore, P. D. Perspective: machines for RNAi. Genes Dev. 19, 517–529 (2005).
Liu, J. et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 (2004).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Pillai, R. S., Artus, C. G. & Filipowicz, W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA 10, 1518–1525 (2004).
Farh, K. K. et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science 310, 1817–1821 (2005).
Bachellerie, J. P., Cavaille, J. & Huttenhofer, A. The expanding snoRNA world. Biochimie 84, 775–790 (2002).
Kiss, T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell 109, 145–148 (2002).
Blum, B., Bakalara, N. & Simpson, L. A model for RNA editing in kinetoplastid mitochondria: 'guide' RNA molecules transcribed from maxicircle DNA provide the edited information. Cell 60, 189–198 (1990).
Stuart, K. D., Schnaufer, A., Ernst, N. L. & Panigrahi, A. K. Complex management: RNA editing in trypanosomes. Trends Biochem. Sci. 30, 97–105 (2005).
Kawasaki, H. & Taira, K. Induction of DNA methylation and gene silencing by short interfering RNAs in human cells. Nature 431, 211–217 (2004).
Matzke, M. A. & Birchler, J. A. RNAi-mediated pathways in the nucleus. Nature Rev. Genet. 6, 24–35 (2005).
Volpe, T. A. et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297, 1833–1837 (2002).
Mochizuki, K. & Gorovsky, M. A. Small RNAs in genome rearrangement in Tetrahymena. Curr. Opin. Genet. Dev. 14, 181–187 (2004).
Butcher, S. E. & Brow, D. A. Towards understanding the catalytic core structure of the spliceosome. Biochem. Soc. Trans. 33, 447–449 (2005).
Gottesman, S. Micros for microbes: non-coding regulatory RNAs in bacteria. Trends Genet. 21, 399–404 (2005).
Zhang, A. et al. Global analysis of small RNA and mRNA targets of Hfq. Mol. Microbiol. 50, 1111–1124 (2003).
Geissmann, T. A. & Touati, D. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 23, 396–405 (2004).
Hopper, A. K. & Phizicky, E. M. tRNA transfers to the limelight. Genes Dev. 17, 162–180 (2003).
Agris, P. F. Decoding the genome: a modified view. Nucleic Acids Res. 32, 223–238 (2004).
Lehmann, K. A. & Bass, B. L. Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry 39, 12875–12884 (2000).
Dragon, F. et al. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417, 967–970 (2002).
Horton, T. L. & Landweber, L. F. Rewriting the information in DNA: RNA editing in kinetoplastids and myxomycetes. Curr. Opin. Microbiol. 5, 620–626 (2002).
Eddy, S. R. Non-coding RNA genes and the modern RNA world. Nature Rev. Genet. 2, 919–929 (2001).
Krek, A. et al. Combinatorial microRNA target predictions. Nature Genet. 37, 495–500 (2005).
Long, M., Betran, E., Thornton, K. & Wang, W. The origin of new genes: glimpses from the young and old. Nature Rev. Genet. 4, 865–875 (2003).
Allen, T. A., Von Kaenel, S., Goodrich, J. A. & Kugel, J. F. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nature Struct. Mol. Biol. 11, 816–821 (2004).
Ofengand, J. Ribosomal RNA pseudouridines and pseudouridine synthases. FEBS Lett. 514, 17–25 (2002).
Liu, B. & Fournier, M. J. Interference probing of rRNA with snoRNPs: a novel approach for functional mapping of RNA in vivo. RNA 10, 1130–1141 (2004).
Tang, T. H. et al. Identification of 86 candidates for small non-messenger RNAs from the archaeon Archaeoglobus fulgidus. Proc. Natl Acad. Sci. USA 99, 7536–7541 (2002).
Dennis, P. P., Omer, A. & Lowe, T. A guided tour: small RNA function in Archaea. Mol. Microbiol. 40, 509–519 (2001).
Renalier, M. H., Joseph, N., Gaspin, C., Thebault, P. & Mougin, A. The Cm56 tRNA modification in Archaea is catalyzed either by a specific 2′-O-methylase, or a C/D sRNP. RNA 11, 1051–1063 (2005).
Ma, X. et al. Pseudouridylation of yeast U2 snRNA is catalyzed by either an RNA-guided or RNA-independent mechanism. EMBO J. 24, 2403–2413 (2005).
Lapeyre, B. & Purushothaman, S. K. Spb1p-directed formation of Gm2922 in the ribosome catalytic center occurs at a late processing stage. Mol. Cell 16, 663–669 (2004).
Kirsebom, L. A. RNase P — a 'Scarlet Pimpernel'. Mol. Microbiol. 17, 411–420 (1995).
Noller, H. F., Hoffarth, V. & Zimniak, L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science 256, 1416–1419 (1992).
Nissen, P., Hansen, J., Ban, N., Moore, P. B. & Steitz, T. A. The structural basis of ribosome activity in peptide bond synthesis. Science 289, 920–930 (2000).
Polacek, N. & Mankin, A. S. The ribosomal peptidyl transferase center: structure, function, evolution, inhibition. Crit. Rev. Biochem. Mol. Biol. 40, 285–311 (2005).
Tran, E., Brown, J. & Maxwell, E. S. Evolutionary origins of the RNA-guided nucleotide-modification complexes: from the primitive translation apparatus? Trends Biochem. Sci. 29, 343–350 (2004).
Deng, W. et al. Organization of the Caenorhabditis elegans small non-coding transcriptome: genomic features, biogenesis, and expression. Genome Res. 16, 20–29 (2006).
Huttenhofer, A., Brosius, J. & Bachellerie, J. P. RNomics: identification and function of small, non-messenger RNAs. Curr. Opin. Chem. Biol. 6, 835–843 (2002).
Tang, T. H. et al. Identification of novel non-coding RNAs as potential antisense regulators in the archaeon Sulfolobus solfataricus. Mol. Microbiol. 55, 469–481 (2005).
Zago, M. A., Dennis, P. P. & Omer, A. D. The expanding world of small RNAs in the hyperthermophilic archaeon Sulfolobus solfataricus. Mol Microbiol. 55, 1812–1828 (2005).
Doudna, J. A. & Cech, T. R. The chemical repertoire of natural ribozymes. Nature 418, 222–228 (2002).
Doudna, J. A. & Rath, V. L. Structure and function of the eukaryotic ribosome: the next frontier. Cell 109, 153–156 (2002).
Storz, G., Altuvia, S. & Wassarman, K. M. An abundance of RNA regulators. Annu. Rev. Biochem. 74, 199–217 (2005).
Hastings, M. L., Ingle, H. A., Lazar, M. A. & Munroe, S. H. Post-transcriptional regulation of thyroid hormone receptor expression by cis-acting sequences and a naturally occurring antisense RNA. J. Biol. Chem. 275, 11507–11513 (2000).
Nordstrom, K. Plasmid R1 — replication and its control. Plasmid 55, 1–26 (2006).
Ambros, V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113, 673–676 (2003).
Lim, L. P. et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433, 769–773 (2005).
Chan, S. W. et al. RNA silencing genes control de novo DNA methylation. Science 303, 1336 (2004).
Aravin, A. A. et al. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5, 337–350 (2003).
Reinhart, B. J. & Bartel, D. P. Small RNAs correspond to centromere heterochromatic repeats. Science 297, 1831 (2002).
Cam, H. P. et al. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nature Genet. 37, 809–819 (2005).
Decatur, W. A. & Fournier, M. J. RNA-guided nucleotide modification of ribosomal and other RNAs. J. Biol. Chem. 278, 695–698 (2003).
Dominski, Z. & Marzluff, W. F. Formation of the 3′ end of histone mRNA. Gene 239, 1–14 (1999).
Marzluff, W. F. Metazoan replication-dependent histone mRNAs: a distinct set of RNA polymerase II transcripts. Curr. Opin. Cell Biol. 17, 274–280 (2005).
Nusinow, D. A. & Panning, B. Recognition and modification of sex chromosomes. Curr. Opin. Genet. Dev. 15, 206–213 (2005).
Chow, J. C., Yen, Z., Ziesche, S. M. & Brown, C. J. Silencing of the mammalian X chromosome. Annu. Rev. Genomics Hum. Genet. 6, 69–92 (2005).
Guthrie, C. & Patterson, B. Spliceosomal snRNAs. Annu. Rev. Genet. 22, 387–419 (1998).
Sharp, P. A. The discovery of split genes and RNA splicing. Trends Biochem. Sci. 30, 279–281 (2005).
Ofengand, J. & Fournier, M. J. in Modification and Editing of RNA (eds Grosjean, H. & Benne, R.) Ch. 12 (American Society for Microbiology Press, Washington DC, 1998).
Acknowledgements
We would like to thank S. Eddy for helpful comments and suggestions and D. Bartel, V. Ambros, R. Bock, M. Terns, L.A. Huber, P. Loidl, N. Polacek and laboratory members from the Division of Genomics and RNomics for critical reading of the manuscript. The work discussed here was supported by an Austrian FWF (Fonds zur Förderung der wissenschaftlichen Forschung) and a German DFG (Deutsche Forschungsgemeinschaft) grant to A.H.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Hüttenhofer, A., Schattner, P. The principles of guiding by RNA: chimeric RNA–protein enzymes. Nat Rev Genet 7, 475–482 (2006). https://doi.org/10.1038/nrg1855
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg1855
This article is cited by
-
Gene editing with CRISPR-Cas12a guides possessing ribose-modified pseudoknot handles
Nature Communications (2021)
-
Genome-wide identification and functional prediction of novel and fungi-responsive lincRNAs in Triticum aestivum
BMC Genomics (2016)
-
Unexpected roles of long non-coding RNAs in cancer biology
Journal of Shanghai Jiaotong University (Science) (2014)
-
Exploration of small non coding RNAs in wheat (Triticum aestivum L.)
Plant Molecular Biology (2012)
-
cDNA library generation from ribonucleoprotein particles
Nature Protocols (2011)