Abstract
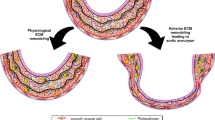
Abdominal aortic aneurysm (AAA) is an important health problem. Elective surgical treatment is recommended on the basis of an individual's risk of rupture, which is predicted by AAA diameter. However, the natural history of AAA differs between patients and a reliable and individual predictor of AAA progression (growth and expansion rates) has not been established. Several circulating biomarkers are candidates for an AAA diagnostic tool. However, they have yet to meet the triad of biomarker criteria: biological plausibility, correlation with AAA progression, and prediction of treatment effect on disease outcome. Circulating levels of markers of extracellular matrix degeneration, such as elastin peptides, aminoterminal propeptide of type III procollagen, elastase–α1-antitrypsin complexes, matrix metalloproteinase 9, cystatin C, plasmin–antiplasmin complexes and tissue plasminogen activator, have been correlated with AAA progression and have biological plausibility. Although studies of these markers have shown promising results, they have not yet led to a clinically applicable biomarker. In future studies, adjustment for initial AAA size, smoking history and the measurement error for determination of AAA size, among other variables, should be taken into account. A large, prospective, standardized, follow-up study will be needed to investigate multiple circulating biomarkers for their potential role in the prediction of AAA progression, followed by a study to investigate the effect of treatment on the circulating levels of biomarkers.
Key Points
-
Abdominal aortic aneurysm has complex pathophysiology
-
Progression of abdominal aortic aneurysm is influenced by many factors
-
The natural history of abdominal aortic aneurysm differs between individual patients
-
An ideal biomarker of abdominal aortic aneurysm progression should have a causal relationship to the disease and should be responsive to intervention
-
Circulating levels of markers of extracellular matrix degeneration (elastin peptides, PIIINP, elastase–A1AT complexes, MMP-9, cystatin C, PAPs and tPA) have biological plausibility and have been reported as potential biomarkers
-
There is a need for a prospective, standardized study to investigate a set of plausible biomarkers for prediction of abdominal aortic aneurysm progression
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
van der Vliet, J. A. & Boll, A. P. Abdominal aortic aneurysm. Lancet 349, 863–866 (1997).
Sakalihasan, N., Limet, R. & Defawe, O. D. Abdominal aortic aneurysm. Lancet 365, 1577–1589 (2005).
Heller, J. A. et al. Two decades of abdominal aortic aneurysm repair: have we made any progress? J. Vasc. Surg. 32, 1091–1100 (2000).
Blankensteijn, J. D. et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N. Engl. J. Med. 352, 2398–2405 (2005).
The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet 352, 1649–1655 (1998).
Glimaker, H. et al. Natural history of patients with abdominal aortic aneurysm. Eur. J. Vasc. Surg. 5, 125–130 (1991).
Kurvers, H. et al. Discontinuous, staccato growth of abdominal aortic aneurysms. J. Am. Coll. Surg. 199, 709–715 (2004).
Fillinger, M. F. et al. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: implications for rupture risk. J. Vasc. Surg. 39, 1243–1252 (2004).
Fillinger, M. F., Marra, S. P., Raghavan, M. L. & Kennedy, F. E. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J. Vasc. Surg. 37, 724–732 (2003).
Golledge, J., Tsao, P. S., Dalman, R. L. & Norman, P. E. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation 118, 2382–2392 (2008).
Hellenthal, F. A., Geenen, I. L., Teijink, J. A., Heeneman, S. & Schurink, G. W. Histological features of human abdominal aortic aneurysm are not related to clinical characteristics. Cardiovasc. Pathol. doi:10.1016/jcarpath.2008.06.014 (2008).
Shimizu, K., Shichiri, M., Libby, P., Lee, R. T. & Mitchell, R. N. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J. Clin. Invest. 114, 300–308 (2004).
Hellenthal, F. A., Buurman, W. A., Wodzig, W. K. & Schurink, G. W. Biomarkers of AAA progression. Part 2: inflammation. Nat. Rev. Cardiol. (in press).
Fleming, T. R. & DeMets, D. L. Surrogate end points in clinical trials: are we being misled? Ann. Intern. Med. 125, 605–613 (1996).
Tardif, J. C., Heinonen, T., Orloff, D. & Libby, P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation 113, 2936–2942 (2006).
Libby, P. & Lee, R. T. Matrix matters. Circulation 102, 1874–1876 (2000).
Dobrin, P. B. & Mrkvicka, R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc. Surg. 2, 484–488 (1994).
Campa, J. S., Greenhalgh, R. M. & Powell, J. T. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis 65, 13–21 (1987).
Davies, M. J. Aortic aneurysm formation: lessons from human studies and experimental models. Circulation 98, 193–195 (1998).
Anidjar, S. et al. Elastase-induced experimental aneurysms in rats. Circulation 82, 973–981 (1990).
Daugherty, A. & Cassis, L. A. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 24, 429–434 (2004).
Libby, P. Changing concepts of atherogenesis. J. Intern. Med. 247, 349–358 (2000).
Holmes, D. R., Liao, S., Parks, W. C. & Thompson, R. W. Medial neovascularization in abdominal aortic aneurysms: a histopathologic marker of aneurysmal degeneration with pathophysiologic implications. J. Vasc. Surg. 21, 761–771 (1995).
Abdul-Hussien, H. et al. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am. J. Pathol. 170, 809–817 (2007).
Jean-Claude, J., Newman, K. M., Li, H., Gregory, A. K. & Tilson, M. D. Possible key role for plasmin in the pathogenesis of abdominal aortic aneurysms. Surgery 116, 472–478 (1994).
Farand, P., Garon, A. & Plante, G. E. Structure of large arteries: orientation of elastin in rabbit aortic internal elastic lamina and in the elastic lamellae of aortic media. Microvasc. Res. 73, 95–99 (2007).
Robert, L., Jacob, M. P. & Fulop, T. Elastin in blood vessels. Ciba Found. Symp. 192, 286–299 (1995).
Sans, M. & Moragas, A. Mathematical morphologic analysis of the aortic medial structure. Biomechanical implications. Anal. Quant. Cytol. Histol. 15, 93–100 (1993).
Cohen, J. R., Mandell, C., Chang, J. B. & Wise, L. Elastin metabolism of the infrarenal aorta. J. Vasc. Surg. 7, 210–214 (1988).
Cohen, J. R., Mandell, C., Margolis, I., Chang, J. & Wise, L. Altered aortic protease and antiprotease activity in patients with ruptured abdominal aortic aneurysms. Surg. Gynecol. Obstet. 164, 355–358 (1987).
Baxter, B. T. et al. Abdominal aortic aneurysms are associated with altered matrix proteins of the nonaneurysmal aortic segments. J. Vasc. Surg. 19, 797–802; discussion 803 (1994).
Brophy, C. M., Reilly, J. M., Smith, G. J. & Tilson, M. D. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann. Vasc. Surg. 5, 229–233 (1991).
Lindholt, J. S., Ashton, H. A., Heickendorff, L. & Scott, R. A. Serum elastin peptides in the preoperative evaluation of abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 22, 546–550 (2001).
Wilson, K. A. et al. The relationship between abdominal aortic aneurysm distensibility and serum markers of elastin and collagen metabolism. Eur. J. Vasc. Endovasc. Surg. 21, 175–178 (2001).
Lindholt, J. S., Vammen, S., Fasting, H., Henneberg, E. W. & Heickendorff, L. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur. J. Vasc. Endovasc. Surg. 20, 281–285 (2000).
Lindholt, J. S., Heickendorff, L., Henneberg, E. W. & Fasting, H. Serum-elastin-peptides as a predictor of expansion of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc Surg. 14, 12–16 (1997).
Lindholt, J. S., Heickendorff, L., Vammen, S., Fasting, H. & Henneberg, E. W. Five-year results of elastin and collagen markers as predictive tools in the management of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 21, 235–240 (2001).
Lindholt, J. S., Jorgensen, B., Fasting, H. & Henneberg, E. W. Plasma levels of plasmin-antiplasmin-complexes are predictive for small abdominal aortic aneurysms expanding to operation-recommendable sizes. J. Vasc. Surg. 34, 611–615 (2001).
He, C. M. & Roach, M. R. The composition and mechanical properties of abdominal aortic aneurysms. J. Vasc. Surg. 20, 6–13 (1994).
Dobrin, P. B., Baker, W. H. & Gley, W. C. Elastolytic and collagenolytic studies of arteries. Implications for the mechanical properties of aneurysms. Arch. Surg. 119, 405–409 (1984).
Murata, K., Motayama, T. & Kotake, C. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis 60, 251–262 (1986).
Thompson, R. W., Geraghty, P. J. & Lee, J. K. Abdominal aortic aneurysms: basic mechanisms and clinical implications. Curr. Probl. Surg. 39, 110–230 (2002).
Satta, J., Haukipuro, K., Kairaluoma, M. I. & Juvonen, T. Aminoterminal propeptide of type III procollagen in the follow-up of patients with abdominal aortic aneurysms. J. Vasc. Surg. 25, 909–915 (1997).
Melkko, J., Niemi, S., Risteli, L. & Risteli, J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clin. Chem. 36, 1328–1332 (1990).
Satta, J., Juvonen, T., Haukipuro, K., Juvonen, M. & Kairaluoma, M. I. Increased turnover of collagen in abdominal aortic aneurysms, demonstrated by measuring the concentration of the aminoterminal propeptide of type III procollagen in peripheral and aortal blood samples. J. Vasc. Surg. 22, 155–160 (1995).
Juvonen, J. et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 17, 2843–2847 (1997).
Antonsen, S. & Wanscher, M. An ELISA for elastase alpha 1-protease inhibitor complexes in human plasma and serum. Scand. J. Clin. Lab. Invest. 53, 145–153 (1993).
Colonnello, J. S. et al. Transient exposure to elastase induces mouse aortic wall smooth muscle cell production of MCP-1 and RANTES during development of experimental aortic aneurysm. J. Vasc. Surg. 38, 138–146 (2003).
Chew, D. K., Orshal, J. M. & Khalil, R. A. Elastase promotes aortic dilation by inhibiting Ca2+ influx into vascular smooth muscle. J. Cardiovasc. Pharmacol. 43, 504–513 (2004).
Vega de Ceniga, M. et al. Search for serum biomarkers associated with abdominal aortic aneurysm growth—a pilot study. Eur. J. Vasc. Endovasc. Surg. 37, 297–299 (2009).
Lindholt, J. S., Jorgensen, B., Klitgaard, N. A. & Henneberg, E. W. Systemic levels of cotinine and elastase, but not pulmonary function, are associated with the progression of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 26, 418–422 (2003).
Miller, F. J., Jr. Aortic aneurysms: it's all about the stress. Arterioscler. Thromb. Vasc Biol. 22, 1948–1949 (2002).
Papadaki, M. et al. Differential regulation of protease activated receptor-1 and tissue plasminogen activator expression by shear stress in vascular smooth muscle cells. Circ. Res. 83, 1027–1034 (1998).
Nelson, K. K. & Melendez, J. A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 37, 768–784 (2004).
Busuttil, R. W., Abou-Zamzam, A. M. & Machleder, H. I. Collagenase activity of the human aorta. A comparison of patients with and without abdominal aortic aneurysms. Arch. Surg. 115, 1373–1378 (1980).
Irizarry, E. et al. Demonstration of interstitial collagenase in abdominal aortic aneurysm disease. J. Surg. Res. 54, 571–574 (1993).
Newman, K. M. et al. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler. Thromb. 14, 1315–1320 (1994).
Curci, J. A., Liao, S., Huffman, M. D., Shapiro, S. D. & Thompson, R. W. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Invest. 102, 1900–1910 (1998).
Mao, D., Lee, J. K., VanVickle, S. J. & Thompson, R. W. Expression of collagenase-3 (MMP-13) in human abdominal aortic aneurysms and vascular smooth muscle cells in culture. Biochem. Biophys. Res. Commun. 261, 904–910 (1999).
Freestone, T. et al. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 15, 1145–1151 (1995).
Wilson, W. R., Schwalbe, E. C., Jones, J. L., Bell, P. R. & Thompson, M. M. Matrix metalloproteinase 8 (neutrophil collagenase) in the pathogenesis of abdominal aortic aneurysm. Br. J. Surg. 92, 828–833 (2005).
Wilson, W. R. et al. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation 113, 438–445 (2006).
Goodall, S., Crowther, M., Hemingway, D. M., Bell, P. R. & Thompson, M. M. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation 104, 304–309 (2001).
Knox, J. B., Sukhova, G. K., Whittemore, A. D. & Libby, P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 95, 205–212 (1997).
Sakalihasan, N., Delvenne, P., Nusgens, B. V., Limet, R. & Lapiere, C. M. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. J. Vasc. Surg. 24, 127–133 (1996).
Hovsepian, D. M. et al. Elevated plasma levels of matrix metalloproteinase-9 in patients with abdominal aortic aneurysms: a circulating marker of degenerative aneurysm disease. J. Vasc. Interv. Radiol. 11, 1345–1352 (2000).
Eugster, T. et al. Aminoterminal propeptide of type III procollagen and matrix metalloproteinases-2 and -9 failed to serve as serum markers for abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 29, 378–382 (2005).
van Laake, L. W. et al. Systemic dilation diathesis in patients with abdominal aortic aneurysms: a role for matrix metalloproteinase-9? Eur. J. Vasc. Endovasc. Surg. 29, 371–377 (2005).
Karlsson, L., Bergqvist, D., Lindbäck, J. & Pärsson, H. Expansion of small-diameter abdominal aortic aneurysms is not reflected by the release of inflammatory mediators IL-6, MMP-9 and CRP in plasma. Eur. J. Vasc. Endovasc. Surg. 37, 420–424 (2009).
Hummel, V. et al. Production of MMPs in human cerebral endothelial cells and their role in shedding adhesion molecules. J. Neuropathol. Exp. Neurol. 60, 320–327 (2001).
Liu, J. et al. Lysosomal cysteine proteases in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 1359–1366 (2004).
Punturieri, A. et al. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J. Exp. Med. 192, 789–799 (2000).
Reddy, V. Y., Zhang, Q. Y. & Weiss, S. J. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L and S by human monocyte-derived macrophages. Proc. Natl Acad. Sci. USA 92, 3849–3853 (1995).
Sukhova, G. K., Shi, G. P., Simon, D. I., Chapman, H. A. & Libby, P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J. Clin. Invest. 102, 576–583 (1998).
Sukhova, G. K. et al. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ. Res. 96, 368–375 (2005).
Shi, G. P. et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J. Clin. Invest. 104, 1191–1197 (1999).
Liu, J. et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis 184, 302–311 (2006).
Lindholt, J. S., Erlandsen, E. J. & Henneberg, E. W. Cystatin C deficiency is associated with the progression of small abdominal aortic aneurysms. Br. J. Surg. 88, 1472–1475 (2001).
Eriksson, P. et al. Genetic approach to the role of cysteine proteases in the expansion of abdominal aortic aneurysms. Br. J. Surg. 91, 86–89 (2004).
Reed, C. H. Diagnostic applications of cystatin C. Br. J. Biomed. Sci. 57, 323–329 (2000).
Wiman, B. & Collen, D. On the mechanism of the reaction between human alpha 2-antiplasmin and plasmin. J. Biol. Chem. 254, 9291–9297 (1979).
Murphy, G., Atkinson, S., Ward, R., Gavrilovic, J. & Reynolds, J. J. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann. NY Acad. Sci. 667, 1–12 (1992).
Werb, Z., Banda, M. J. & Jones, P. A. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J. Exp. Med. 152, 1340–1357 (1980).
Lindholt, J. S., Jorgensen, B., Shi, G. P. & Henneberg, E. W. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 25, 546–551 (2003).
Sofi, F. et al. High levels of homocysteine, lipoprotein (a) and plasminogen activator inhibitor-1 are present in patients with abdominal aortic aneurysm. Thromb. Haemost. 94, 1094–1098 (2005).
Adam, D. J., Ludlam, C. A., Ruckley, C. V. & Bradbury, A. W. Coagulation and fibrinolysis in patients undergoing operation for ruptured and nonruptured infrarenal abdominal aortic aneurysms. J. Vasc. Surg. 30, 641–650 (1999).
Dahlback, B. Blood coagulation. Lancet 355, 1627–1632 (2000).
Kolbel, T., Strandberg, K., Mattiasson, I., Stenflo, J. & Lindblad, B. Activated protein C–protein C inhibitor complex: a new biological marker for aortic aneurysms. J. Vasc. Surg. 43, 935–939 (2006).
Kolbel, T. et al. Activated protein C–protein C inhibitor complex in patients with abdominal aortic aneurysms: is it associated with diameter and growth rate? Vasc. Endovasc. Surg. 42, 135–140 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hellenthal, F., Buurman, W., Wodzig, W. et al. Biomarkers of AAA progression. Part 1: extracellular matrix degeneration. Nat Rev Cardiol 6, 464–474 (2009). https://doi.org/10.1038/nrcardio.2009.80
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2009.80
This article is cited by
-
Microskeletal stiffness promotes aortic aneurysm by sustaining pathological vascular smooth muscle cell mechanosensation via Piezo1
Nature Communications (2022)
-
AVE0991, a nonpeptide angiotensin-(1–7) mimic, inhibits angiotensin II–induced abdominal aortic aneurysm formation in apolipoprotein E knockout mice
Journal of Molecular Medicine (2020)
-
Smooth Muscle Sirtuin 1 Blocks Thoracic Aortic Aneurysm/Dissection Development in Mice
Cardiovascular Drugs and Therapy (2020)
-
Meta-analysis of the growth rates of abdominal aortic aneurysm in the Chinese population
BMC Cardiovascular Disorders (2019)
-
An in vitro method to keep human aortic tissue sections functionally and structurally intact
Scientific Reports (2018)