Abstract
The goal of individualized drug therapy requires physicians to be able to accurately predict an individual's response to a drug. Both genetic and environmental factors are known to influence drug response. 'Pharmacogenetics' is the study of the role of inheritance in variation in drug response phenotypes. Pharmacogenetics is now moving genome-wide to become 'pharmacogenomics', resulting in the recognition of novel biomarkers for individual variation in drug response. This article reviews the development, promise and challenges facing pharmacogenomics, using examples of drugs used to treat or prevent cardiovascular disease.
Key Points
-
Genetic variation has an important role in explaining individual variation in drug response, and genetic analysis could help us provide appropriate care to individual patients
-
Historically, this type of study has been confined to candidate genes for proteins involved in known pharmacokinetic and pharmacodynamic pathways for the drug—that is, 'pharmacogenetics'
-
Pharmacokinetic pathways influence the concentration of a drug reaching its target
-
Pharmacodynamic pathways influence the drug target itself and signaling pathways downstream of the drug target
-
Our ability to perform genome-wide studies across the entire human genome has transformed 'pharmacogenetics' into 'pharmacogenomics'
-
Clinical trials are needed to assess the clinical utility of genotyping in drug therapy and to justify the cost of genetic testing in clinical practice
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Scriver, C. R. & Childs, B. (eds) Garrod's Inborn Factors in Disease (Oxford University Press, New York, 1989). [Oxford Monographs on Medical Genetics Vol. 16].
Motulsky, A. G. Drug reactions enzymes, and biochemical genetics. JAMA 165, 835–837 (1957).
Vesell, E. S. & Page, J. G. Genetic control of drug levels in man: antipyrine. Science 161, 72–73 (1968).
Kalow, W. & Gunn, D. R. The relationship between dose of succinylcholine and duration of apnea in man. J. Pharmacol. Exp. Ther. 120, 203–214 (1957).
Kalow, W. & Gunn, D. R. Some statistical data on atypical cholinesterase of human serum. Ann. Hum. Genet. 23, 239–250 (1959).
Lockridge, O. in Pharmacogenetics of Drug Metabolism: International Encyclopedia of Pharmacology and Therapeutics (ed. Kalow, W.) 15–50 (Pergamon Press, New York, 1992).
Pearson, T. A. & Manolio, T. A. How to interpret a genome-wide association study. JAMA 299, 1335–1344 (2008).
Reidenberg, M. M., Drayer, D. E., Levy, M. & Warner, H. Polymorphic acetylation procainamide in man. Clin. Pharmacol. Ther. 17, 722–730 (1975).
Perry, H. M. Jr, Tan, E. M., Carmody, S. & Sakamoto, A. Relationship of acetyl transferase activity to antinuclear antibodies and toxic symptoms in hypertensive patients treated with hydralazine. J. Lab. Clin. Med. 76, 114–125 (1970).
Price Evans, D. A. in Pharmacogenetics of Drug Metabolism: International Encyclopedia of Pharmacology and Therapeutics (ed. Kalow, W.) 95–178 (Pergamon Press, New York, 1992).
Eichelbaum, M., Spannbrucker, N., Steincke, B. & Dengler, H. J. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur. J. Clin. Pharmacol. 16, 183–187 (1979).
Lennard, M. S. et al. Oxidation phenotype—a major determinant of metoprolol metabolism and response. N. Engl. J. Med. 307, 1558–1560 (1982).
Gonzalez, F. J. et al. Human debrisoquine 4-hydroxylase (P450IID1): cDNA and deduced amino acid sequence and assignment of the CYP2D locus of chromosome 22. Genomics 2, 174–179 (1988).
Kimura, S., Umeno, M., Skoda, R. C., Meyer, U. A. & Gonzalez, F. J. The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am. J. Hum. Genet. 45, 889–904 (1989).
Johansson, I. et al. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc. Natl Acad. Sci. USA 90, 11825–11829 (1993).
Akulli, E. et al. Frequent distribution of ultrarapid metabolizers of debrisoquine in an Ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J. Pharmacol. Exp. Ther. 278, 441–446 (1996).
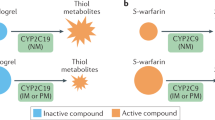
Mega, J. L. et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360, 354–362 (2008).
Roger, V. L. et al. Trends in heart failure incidence and survival in a community-based population. JAMA 292, 344–350 (2004).
Bardy G. H. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 352, 225–237 (2005).
The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N. Engl. J. Med. 344, 1659–1667 (2001).
Taylor, M. R. Pharmacogenetics of the human beta-adrenergic receptors. Pharmacogenomics J. 7, 29–37 (2007).
Moore, J. D., Mason, D. A., Green, S. A., Hsu, J. & Liggett, S. B. Racial differences in the frequencies of cardiac beta(1)-adrenergic receptor polymorphisms: analysis of c145A>G and c1165G>C. Hum. Mutat. 14, 271 (1999).
Liggett, S. B. et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc. Natl Acad. Sci. USA 103, 11288–11293 (2006).
Small, K. M., Forbes, S. L., Rahman, F. F., Bridges, K. M. & Liggett, S. B. A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J. Biol. Chem. 275, 23059–23064 (2000).
Small, K. M., Wagoner, L. E., Levin, A. M., Kardia, S. L. & Liggett, S. B. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N. Engl. J. Med. 347, 1135–1142 (2002).
Liggett, S. B. et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nat. Med. 14, 510–517 (2008).
Budnitz, D. S., Shehab, N., Kegler, S. R. & Richards, C. L. Medication use leading to emergency department visits for adverse drug events in older adults. Ann. Intern. Med. 147, 755–765 (2007).
O'Reilly, R. A., Aggeler, P. M., Hoag, M. S., Leong, L. S. & Kropatkin, M. L. Hereditary transmission of exceptional resistance to coumarin anticoagulant drugs. The first reported kindred. N. Engl. J. Med. 271, 809–815 (1964).
Daly, A. K. & King, B. P. Pharmacogenetics of oral anticoagulants. Pharmacogenetics 13, 247–252 (2003).
Aithal, G. P., Day, C. P., Kesteven, P. J. & Daly, A. K. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353, 717–719 (1999).
Rost, S. et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427, 537–541 (2004).
Rieder, M. J. et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 352, 2285–2293 (2005).
Schwarz, U. I. et al. Genetic determinants of response to warfarin during initial anticoagulation. N. Engl. J. Med. 358, 999–1008 (2008).
Lesko, L. J. The critical path of warfarin dosing: finding an optimal dosing strategy using pharmacogenetics. Clin. Pharmacol. Ther. 84, 301–303 (2008).
International Warfarin Pharmacogenetics Consortium et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 360, 753–764 (2009).
The SEARCH Collaborative Group et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N. Engl. J. Med. 359, 789–799 (2008).
Mangravite, L. M. & Krauss, R. M. Pharmacogenomics of statin response. Curr. Opin. Lipidol. 18, 409–414 (2007).
Zuccaro, P. et al. Tolerability of statins is not linked to CYP450 polymorphisms, but reduced CYP2D6 metabolism improves cholesteraemic response to simvastatin and fluvastatin. Pharmacol. Res. 55, 310–317 (2007).
Wang, L. & Weinshilboum, R. M. Pharmacogenomics: candidate gene identification, functional validation and mechanisms. Hum. Mol. Genet. 17, R174–R179 (2008).
Li, L. et al. Gemcitabine and cytosine arabinoside cytotoxicity: association with lymphoblastoid cell expression. Cancer Res. 68, 7050–7058 (2008).
Pei, H. et al. FKBP51 acts as a scaffolding protein to regulate Akt phosphorylation. Cancer Cell (in press).
Weinstein, J. N. Spotlight on molecular profiling: 'Integromic' analysis of the NCI-60 cancer cell lines. Mol. Cancer Ther. 5, 2601–2605 (2006).
Hughes, D. A. & Pirmohamed, M. Warfarin pharmacogenetics: economic considerations. Pharmacoeconomics 25, 899–902 (2007).
Eckman, M. H., Rosand, J., Greenberg, S. M. & Gage, B. F. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann. Intern. Med. 150, 73–83 (2009).
Hudson, K. L., Holohan, M. K. & Collins, F. S. Keeping pace with the times—the Genetic Information Nondiscrimination Act of 2008. N. Engl. J. Med. 358, 2661–2663 (2008).
Jones, M. Francis Collins Addresses State of Personalized Medicine. GenomeWeb [online], (2009).
Acknowledgements
Supported in part by HL 84904 (Heart Failure Clinical Research Network), a Marie Ingalls Cardiovascular Career Development Award (N. L. Pereira), a PhRMA Foundation “Center of Excellence in Clinical Pharmacology” Award (R. M. Weinshilboum), and NIH grants UL1RR24150 (N. L. Pereira), R01 GM28157 (R. M. Weinshilboum), R01 CA132780 (R. M. Weinshilboum) and U01 GM61388 (The Pharmacogenetics Research Network). We thank L. Wussow for her assistance with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pereira, N., Weinshilboum, R. Cardiovascular pharmacogenomics and individualized drug therapy. Nat Rev Cardiol 6, 632–638 (2009). https://doi.org/10.1038/nrcardio.2009.154
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2009.154
This article is cited by
-
De-escalation from Prasugrel or Ticagrelor to Clopidogrel in Patients with Acute Coronary Syndrome Managed with Percutaneous Coronary Intervention: An Updated Meta-analysis of Randomized Clinical Trials
American Journal of Cardiovascular Drugs (2022)
-
Ex Ante Economic Evaluation of Arg389 Genetically Targeted Treatment with Bucindolol versus Empirical Treatment with Carvedilol in NYHA III/IV Heart Failure
American Journal of Cardiovascular Drugs (2021)
-
International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines
The Journal of nutrition, health and aging (2021)
-
Genetics of dilated cardiomyopathy: practical implications for heart failure management
Nature Reviews Cardiology (2020)
-
Exercise training response heterogeneity: physiological and molecular insights
Diabetologia (2017)